Water is essential for life as we know it and we are all familiar with the sight of water in the atmosphere and on the surface of the earth. But where is the water deep down, beyond the crust?
The mantle (the part of the earth between the crust and the core) is primarily composed of iron-bearing magnesium silicates which undergo a number of phase transitions with depth. At the transition between the upper and lower mantle, olivine transforms into wadsleyite and this mineral has received interest due to its potential for hosting hydrogen, which is termed water in the realm of the inner-Earth. If fully hydrated the amount of hydrogen potentially stored in this mineral is four times the amount present in the oceans and the atmosphere.
When a hydrogen atom is incorporated into the mineral it is balanced by the removal of a magnesium cation, this produces several possible locations for the incorporation of the hydrogen in the vacancy, as well as different possible ordering of the vacancy in the structure. To solve this complex problem Stephen Wimperis and Sharon Ashbrook have teamed up and used the developments of high-field NMR and sophisticated experimental methods to study the ‘difficult’ NMR nuclei of 25Mg and 17O within hydrated wadsleyite. In addition to the experimental approach, they have also carried out extensive DFT calculations.
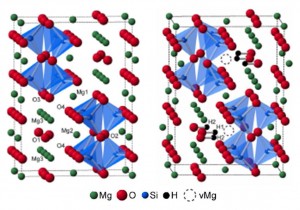
Anhydrous (left) and one of the hypothetical ordered structures for fully-hydrated wadsleyite (right).
Most of the hydrogen was found to be located on the O1 site with the substitution charge balanced by Mg3 cation vacancies. A smaller amount of hydrogen was also found to be present in slightly higher energy defect sites with different proton arrangements or centred at different cation vacancies.
This study demonstrates the benefits of using a combined computational and experimental approach to reveal the location of hydrogen within wadsleyite. The use of this multinuclear NMR approach can be used to investigate a wide range of other silicate phases which should lead to a better understanding of the locations and distribution of hydrogen in the Earth’s mantle.
For more, read this ‘HOT’ Chemical Science article in full:
Water in the Earth’s Mantle: A Solid-State NMR Study of Hydrous Wadsleyite
John M. Griffin, Andrew J. Berry, Daniel J. Frost, Stephen Wimperis and Sharon E. Ashbrook
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC21892A
Comments Off on Where is the Water?












-429---NS_tcm18-221958.jpg)

