This month, Chemical Science is delighted to welcome Zhenan Bao as Associate Editor in the area of organic materials. Zhenan received her PhD from the University of Chicago in 1995, and after a spell at Lucent Technologies’ Bell Labs, joined the Department of Chemical Engineering at Stanford University in 2004. She was appointed to her present position as Professor in 2012.
This month, Chemical Science is delighted to welcome Zhenan Bao as Associate Editor in the area of organic materials. Zhenan received her PhD from the University of Chicago in 1995, and after a spell at Lucent Technologies’ Bell Labs, joined the Department of Chemical Engineering at Stanford University in 2004. She was appointed to her present position as Professor in 2012.
Zhenan was awarded the Beilby Medal and Prize by the Royal Society of Chemistry in 2009, for her contributions and discoveries in the field of organic semiconductors. These included demonstrating that conjugated polymers can produce high mobilities of charge carriers when self-assembled using solution deposition.
She also featured in Thomson Reuter’s ‘Top 100 Materials Scientists’ report in 2011, which identified researchers with the highest citation counts for papers published in 2000–2010.
The Bao Group’s research utilizes the basic principles in chemistry, physics and material sciences to enable novel applications and development of flexible, stretchable electronics and energy devices. Current research projects within the group include organic semi-conductor design, organic and carbon solar cells, and electronic skin.
The group has published work in a number of Royal Society of Chemistry journals, and you can read more about it in the following articles:
Probing interfacial molecular packing in TIPS-pentacene organic semiconductors by Surface enhanced Raman Scattering
Jie Xu, Ying Diao, Dongshan Zhou, Yisha Mao, Gaurav Giri, Wei Chen, Nan Liu, Stefan C B Mannsfeld, Gi Xue and Zhenan Bao
J. Mater. Chem. C, 2014, Accepted Manuscript, DOI: 10.1039/C3TC32581D
Sequentially solution-processed, nanostructured polymer photovoltaics using selective solvents
Do Hwan Kim, Jianguo Mei, Alexander L. Ayzner, Kristin Schmidt, Gaurav Giri, Anthony L. Appleton, Michael F. Toney and Zhenan Bao
Energy Environ. Sci., 2014, 7, 1103-1109, DOI: 10.1039/C3EE43541E, Paper
A review of fabrication and applications of carbon nanotube film-based flexible electronics
Steve Park, Michael Vosguerichian and Zhenan Bao
Nanoscale, 2013, 5, 1727-1752, DOI: 10.1039/C3NR33560G
Confined organization of fullerene units along high polymer chains
Lei Fang, Peng Liu, Benjamin R. Sveinbjornsson, Sule Atahan-Evrenk, Koen Vandewal, Sílvia Osuna, Gonzalo Jiménez-Osés, Supriya Shrestha, Gaurav Giri, Peng Wei, Alberto Salleo, Alán Aspuru-Guzik, Robert H. Grubbs, K. N. Houk and Zhenan Bao
J. Mater. Chem. C, 2013, 1, 5747-5755, DOI: 10.1039/C3TC31158A
5,11-Conjugation-extended low-bandgap anthradithiophene-containing polymer exhibiting enhanced thin-film order and field-effect mobility
Ying Jiang, Jianguo Mei, Alexander L. Ayzner, Michael F. Toney and Zhenan Bao
Chem. Commun., 2012, 48, 7286-7288, DOI: 10.1039/C2CC32473C
Impact of regioregularity on thin-film transistor and photovoltaic cell performances of pentacene-containing polymers
Ying Jiang, Sanghyun Hong, Joon Hak Oh, Rajib Mondal, Toshihiro Okamoto, Eric Verploegen, Michael F. Toney, Michael D. McGehee and Zhenan Bao
J. Mater. Chem., 2012, 22, 4356-4363, DOI: 10.1039/C2JM15483H
Graphene–sponges as high-performance low-cost anodes for microbial fuel cells
Xing Xie, Guihua Yu, Nian Liu, Zhenan Bao, Craig S. Criddle and Yi Cui
Energy Environ. Sci., 2012, 5, 6862-6866, DOI: 10.1039/C2EE03583A
Zhenan is now accepting submissions to Chemical Science in the area of organic materials. Submit your high-impact research to her Editorial Office.
Comments Off on Chemical Science welcomes Zhenan Bao as Associate Editor












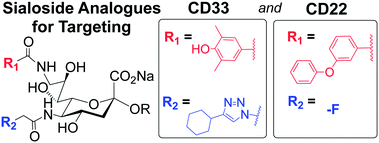

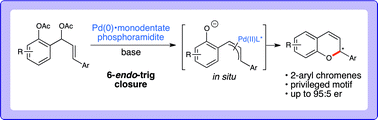

 Have you seen the most-read Chemical Science articles from 2013?
Have you seen the most-read Chemical Science articles from 2013?