The 25 most-downloaded Chemical Science articles in the second quarter of 2015 were as follows:
Copper-catalyzed intermolecular C(sp3)–H bond functionalization towards the synthesis of tertiary carbamates
Prasanna Kumara Chikkade, Yoichiro Kuninobu and Motomu Kanai
DOI: 10.1039/C5SC00238A, Edge Article
Palladium(0) NHC complexes: a new avenue to highly efficient phosphorescence
Adam F. Henwood, Mathieu Lesieur, Ashu K. Bansal, Vincent Lemaur, David Beljonne, David G. Thompson, Duncan Graham, Alexandra M. Z. Slawin, Ifor D. W. Samuel, Catherine S. J. Cazin and Eli Zysman-Colman
DOI: 10.1039/C4SC03914A, Edge Article
Rh-catalyzed decarbonylation of conjugated ynones via carbon–alkyne bond activation: reaction scope and mechanistic exploration via DFT calculations
Alpay Dermenci, Rachel E. Whittaker, Yang Gao, Faben A. Cruz, Zhi-Xiang Yu and Guangbin Dong
DOI: 10.1039/C5SC00584A, Edge Article
Sulfonyl fluorides as privileged warheads in chemical biology
Arjun Narayanan and Lyn H. Jones
DOI: 10.1039/C5SC00408J, Perspective
Computational discovery and experimental verification of tyrosine kinase inhibitor pazopanib for the reversal of memory and cognitive deficits in rat model neurodegeneration
Yongliang Yang, Guohui Li, Dongyu Zhao, Haoyang Yu, Xiliang Zheng, Xiangda Peng, Xiaoe Zhang, Ting Fu, Xiaoqing Hu, Mingshan Niu, Xuefei Ji, Libo Zou and Jin Wang
DOI: 10.1039/C4SC03416C, Edge Article
A divergent route to core- and peripherally functionalized diazacoronenes that act as colorimetric and fluorescence proton sensors
Bo He, Jing Dai, Danylo Zherebetskyy, Teresa L. Chen, Benjamin A. Zhang, Simon J. Teat, Qichun Zhang, Linwang Wang and Yi Liu
DOI: 10.1039/C5SC00304K, Edge Article
Spatial imaging of carbon reactivity centers in Pd/C catalytic systems
E. O. Pentsak, A. S. Kashin, M. V. Polynski, K. O. Kvashnina, P. Glatzel and V. P. Ananikov
DOI: 10.1039/C5SC00802F, Edge Article
Chemical assay-guided natural product isolation via solid-supported chemodosimetric fluorescent probe
Hongjun Jeon, Chaemin Lim, Ji Min Lee and Sanghee Kim
DOI: 10.1039/C5SC00360A, Edge Article
Very bright mechanoluminescence and remarkable mechanochromism using a tetraphenylethene derivative with aggregation-induced emission
Bingjia Xu, Jiajun He, Yingxiao Mu, Qiangzhong Zhu, Sikai Wu, Yifan Wang, Yi Zhang, Chongjun Jin, Changcheng Lo, Zhenguo Chi, Alan Lien, Siwei Liu and Jiarui Xu
DOI: 10.1039/C5SC00466G, Edge Article
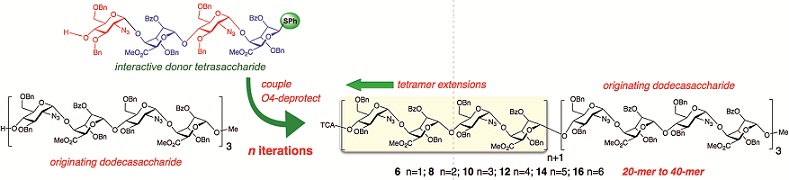
Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry
Swati S. Nigudkar and Alexei V. Demchenko
DOI: 10.1039/C5SC00280J, Perspective
Combination of Ru(II) complexes and light: new frontiers in cancer therapy
Cristina Mari, Vanessa Pierroz, Stefano Ferrari and Gilles Gasser
DOI: 10.1039/C4SC03759F, Perspective
Enantioselective synthesis of bicyclo[3.n.1]alkanes by chiral phosphoric acid-catalyzed desymmetrizing Michael cyclizations
Alan R. Burns, Amaël G. E. Madec, Darryl W. Low, Iain D. Roy and Hon Wai Lam
DOI: 10.1039/C5SC00753D, Edge Article
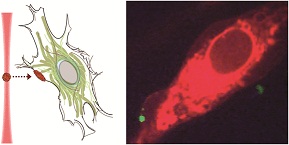
Ratiometric detection of pH fluctuation in mitochondria with a new fluorescein/cyanine hybrid sensor
Yuncong Chen, Chengcheng Zhu, Jiajie Cen, Yang Bai, Weijiang He and Zijian Guo
DOI: 10.1039/C4SC04021J, Edge Article
Enantioselective and diastereoselective azo-coupling/iminium-cyclizations: a unified strategy for the total syntheses of (−)-psychotriasine and (+)-pestalazine B
Qi Li, Tingting Xia, Licheng Yao, Haiteng Deng and Xuebin Liao
DOI: 10.1039/C5SC00338E, Edge Article
Iron(II)-catalyzed asymmetric intramolecular olefin aminochlorination using chloride ion
Cheng-Liang Zhu, Jun-Shan Tian, Zhen-Yuan Gu, Guo-Wen Xing and Hao Xu
DOI: 10.1039/C5SC00221D, Edge Article
Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics
Eric T. Hoke, Daniel J. Slotcavage, Emma R. Dohner, Andrea R. Bowring, Hemamala I. Karunadasa and Michael D. McGehee
DOI: 10.1039/C4SC03141E, Edge Article
Evaluating metal–organic frameworks for natural gas storage
Jarad A. Mason, Mike Veenstra and Jeffrey R. Long
DOI: 10.1039/C3SC52633J, Perspective
Enhancing H2 evolution performance of an immobilised cobalt catalyst by rational ligand design
Janina Willkomm, Nicoleta M. Muresan and Erwin Reisner
DOI: 10.1039/C4SC03946G, Edge Article
Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide
David S. Surry and Stephen L. Buchwald
DOI: 10.1039/C0SC00331J, Perspective
Diverse reactivity of a tricoordinate organoboron L2PhB: (L = oxazol-2-ylidene) towards alkali metal, group 9 metal, and coinage metal precursors
Lingbing Kong, Rakesh Ganguly, Yongxin Li and Rei Kinjo
DOI: 10.1039/C5SC00404G, Edge Article
Is a polymer semiconductor having a “perfect” regular structure desirable for organic thin film transistors?
Wei Hong, Shaoyun Chen, Bin Sun, Mark A. Arnould, Yuezhong Meng and Yuning Li
DOI: 10.1039/C5SC00843C, Edge Article
Palladium-catalyzed cross-coupling of α-bromocarbonyls and allylic alcohols for the synthesis of α-aryl dicarbonyl compounds
Yang Yu and Uttam K. Tambar
DOI: 10.1039/C5SC00505A, Edge Article
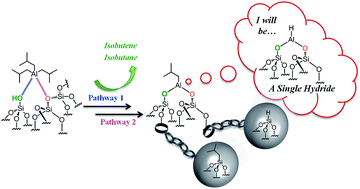
Charging and discharging at the nanoscale: Fermi level equilibration of metallic nanoparticles
Micheál D. Scanlon, Pekka Peljo, Manuel A. Méndez, Evgeny Smirnov and Hubert H. Girault
DOI: 10.1039/C5SC00461F, Perspective
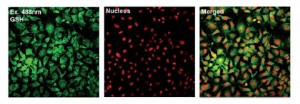
A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging
Zhiqian Guo, SeongWon Nam, Sungsu Park and Juyoung Yoon
DOI: 10.1039/C2SC20540H, Edge Article
Virtual screening for high affinity guests for synthetic supramolecular receptors
William Cullen, Simon Turega, Christopher A. Hunter and Michael D. Ward
DOI: 10.1039/C5SC00534E, Edge Article
Chemical Science is the Royal Society of Chemistry’s flagship journal, publishing research articles of exceptional significance and high-impact reviews from across the chemical sciences. It has been Gold Open Access since January 2015.
Submit your exceptional research to Chemical Science today!
Stay up to date with Chemical Science
Be among the first to hear about the newest articles being published – Sign-up to our journal news alert to receive information about most read articles, journal news, as well as calls for papers and invitations.
Comments Off on Top 25 Chemical Science articles April–June 2015