One of my highlights from Challenges in Organic Chemistry and Chemical Biology (ISACS7) was Tom Muir’s talk on protein ligation. His work is truly inspiring so I caught up with him to find out how a boy from bonnie Scotland became a world leader in protein engineering.
Here is an excerpt from that interview; the full interview is published in Chemistry World.
Protein Power
 Tom Muir, professor of chemistry and molecular biology, Princeton University, US, is an expert in protein engineering and its application to studying cellular signalling networks. His lab has developed a suite of chemistry-driven tools for studying the structure and function of proteins in the test tube and in live cells. In addition, his laboratory employs cutting edge methods in protein engineering (computational protein design and directed evolution), structural biology (NMR spectroscopy and x-ray crystallograghy) and cell biology (imaging).
Tom Muir, professor of chemistry and molecular biology, Princeton University, US, is an expert in protein engineering and its application to studying cellular signalling networks. His lab has developed a suite of chemistry-driven tools for studying the structure and function of proteins in the test tube and in live cells. In addition, his laboratory employs cutting edge methods in protein engineering (computational protein design and directed evolution), structural biology (NMR spectroscopy and x-ray crystallograghy) and cell biology (imaging).
Tom Muir is Chemical Science‘s new Associate Editor for Chemical Biology. Find out more >
What led you to a career in science? Were you interested in science from an early age?
I would be lying if I said I was deeply passionate about it when I was in high school, but I could always do it and it was the path of least resistance into university. It was when I got to university that I first realised that I was lucky enough to have chosen a major that I really liked. I made some great friends and we collectively discovered the subject together. I loved the logic of chemistry.
You studied for your undergraduate degree and PhD in Edinburgh. How did you find the move to the US? What do you think are the main differences between practising science in the US and the UK?
I knew within weeks of arriving in the US that I was never coming back. I loved it! I moved to Southern California and, as someone coming from the west of Scotland, I found it quite agreeable. I was at The Scripps Research Institute in La Jolla when I was a post doc and the sense of anything’s possible in science there was pervasive and infectious; the penny truly dropped in terms of what it means to be a research scientist. The ‘can do’ attitude that I experienced knocked me out. Sitting on the beach didn’t hurt either!
You started out as an organic chemist, but your work now combines chemistry with biochemistry and cell biology. How did you make the transition into this interdisciplinary research area?
The move to chemical biology wasn’t part of a grand plan. I have always felt like I am on a boat being blown in different directions on a lake. Mainly, I’ve been very lucky in the people that I have interacted with, both mentors and collaborators.
I have to thank particularly my PhD supervisor at Edinburgh, Bob Ramage, who is an amazing organic chemist and whose approach to the subject was rigorous and forward looking. He appreciated just how much more chemistry had to offer biologists, molecular biologists specifically, and he set up the post doc position for me in San Diego. I worked with Stephen Kent, who was also an amazing mentor, and I was fortunate to be in his lab during a critical period in the development of modern protein chemistry. As I learned more about biology, I absolutely bought into the importance of chemistry in solving much more complicated biological problems.
Then I moved to Rockefeller as an assistant professor. Rockefeller has many amazing biologists, probably unequalled. I had the opportunity to talk to all these luminaries and they introduced me to problems that I hadn’t even thought about. They once again highlighted the huge role that chemistry has to play, opening new doors for me.
Your research revolves around proteins and how they work. What is it about this particular type of biomolecule that fascinates you?
I am staggered by how byzantine they are. They are incredibly complicated machines. It is almost like peeling back layers of an onion: you think you understand one layer and you peel it back and there is a whole other layer of complexity underneath. With each layer, you get closer and closer to physics. It always amazes me how complicated their regulation is, how many different ways they can be controlled and how many different types of chemistry they have evolved to catalyse reactions and to recognise other types of biological molecules. But I’ve always thought of them as big organic molecules and therefore it seems natural to me that organic chemists should be studying them.
Have you never found their complexity daunting?
I always find it daunting, but I think tackling daunting tasks is exactly what academics should be doing. It is easier, because it is safe, to work on problems that are in a sense crumbs off the big table. But chemists should have a chip on this table, we should be working on problems that make us throw our hands in the air and shout ‘I’ll never figure this out!’ We have to try. At some point, everything was daunting until someone figured it out. I am not saying that I am going to be the one to figure out say epigenetics, but if nobody tries, it will forever remain a mystery. Yes, I feel daunted but that inspires, rather than scares, me.
Read more in Chemistry World >


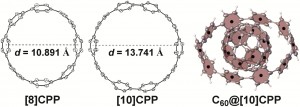









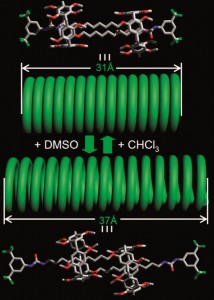
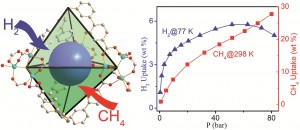
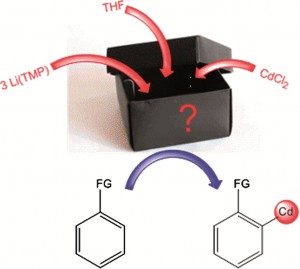

![molecules Graphical abstract: Experimental and quantum chemical characterization of the water oxidation cycle catalysed by [RuII(damp)(bpy)(H2O)]2+](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2SC20399E) Catalysis is all around us, from the enzymatic reactions in our body to the synthesis of fuels and medicines. Quite rightly, it forms an important part of
Catalysis is all around us, from the enzymatic reactions in our body to the synthesis of fuels and medicines. Quite rightly, it forms an important part of 