That’s it. British summertime is officially over. So to help us through the cold, dark winter, I’ve collected together some of the hottest Edge articles published in October.
That’s it. British summertime is officially over. So to help us through the cold, dark winter, I’ve collected together some of the hottest Edge articles published in October.
Bright results
Oligomeric tandem terpyridyl platinum(II) complexes display spectacular solvent dependent emission properties and self-aggregating behaviour. What factors govern this strong emissive property? Find out in Chi-Ming Che’s Edge Article.
Catalytic water oxidation
Robert Crabtree and colleagues report a new method for generating an amorphous electrodeposited material, principally consisting of iridium and oxygen, which is a robust and long-lived catalyst for water oxidation, when driven electrochemically.
Forming C-C bonds
A novel method for the direct, amine-catalysed, highly enantioselective α-alkylation of aldehydes has been discovered by Claudio Palomo and colleagues. Find out more about the reaction conditions and mechanism.
Extending cyclopropenium activation
Geminal dichlorocyclopropenes rapidly and efficiently convert oximes to amides at room temperature, with reactivity that far surpasses other organic-based promoters, report Tristan Lambert and colleagues in their Edge article.
Understanding aerobic oxidation
Experimental and computational data provide direct evidence for two parallel mechanistic pathways for O2 insertion into a Pd–H bond, claim Shannon Stahl and colleagues. Assess the evidence for yourself in their Edge Article.
Gauging electronic dissymmetry
What kinds of environments give rise to effective levels of electronic dissymmetry in metal complexes? Seth Brown and colleagues investigate the behaviour of a series of tetrasubstituted biphenolates with substituents of varying electronic and steric character and shed light on the origins of stereoselectivity in these complexes. Read more…
Easy access to polycyclic ring systems
Daniel Seidel and colleagues report a new 1,6-annulation reaction for azomethine ylides which could find widespread use in alkaloid synthesis.
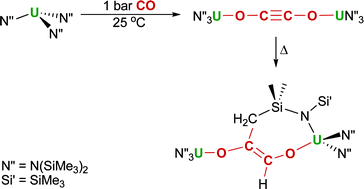
Catalytic nitrene transfer
Alan Heyduk and colleagues report on the catalytic formation of carbodiimide using zirconium(IV) bearing a redox-active ligand.
If you like the sound of these articles, subscribe to the Chemical Science e-alert to receive details of the latest issues. And if you have some hot science to report, submit to Chemical Science.