Bioluminescent enzymes (luciferases) generate light via the oxidation of small molecule luciferins. The process is highly specific and accurate even at heterogeneous environment. Luciferin-luciferases based imaging technique is highly appreciated for specificity in tracking cell movements, cell proliferation, and numerous other features in living organisms.
Imaging of in-depth organ tissues requires emission at NIR region for effective penetration through tissue layer. There existed a big gap in successful synthesis followed by appropriate multiplexed imaging application of bioluminescent pairs. Researchers from University of California, Irvine recently developed a unique class of orthogonal, NIR emitting luciferins that could promise more accessible, long-wavelength bioluminescent pairs for in-vivo imaging.
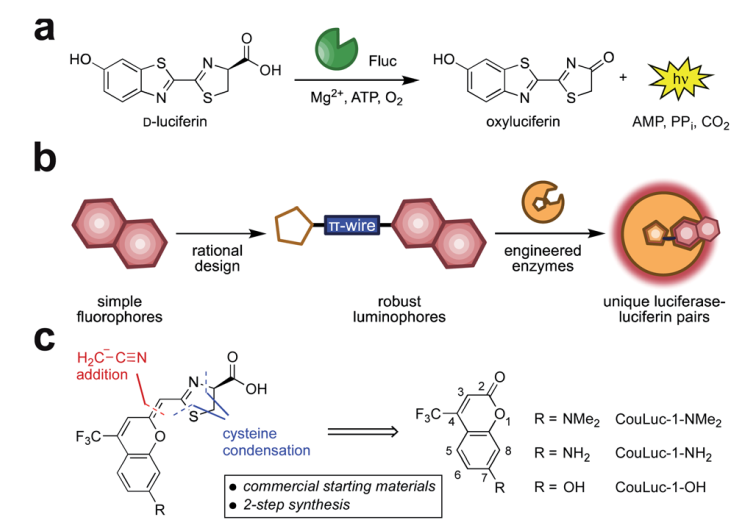
Fig. 1 Red-emitting orthogonal bioluminescent probes designed from fluorophores. (a) D-Luciferin is oxidized by firefly luciferase (Fluc) to produce oxyluciferin and a photon of light. (b) Coumarin fluorophores were used as templates for red-shifted luciferins. (c) Retrosynthetic analysis of the CouLuc-1 analogs.
The authors focused on a new class of luciferins (CouLuc-1s) comprising both an elongated pi-system and a 4-tri-fluoromethylcoumarin unit (Fig 1). The synthesis follows two-step route to bridge the fluorescent coumarin heterocycle with the key thiazoline unit necessary for luciferin bioluminescence. The small size of the coumarin core require only minimal enzyme engineering to identify complementary luciferases that were identified via a parallel engineering approach.
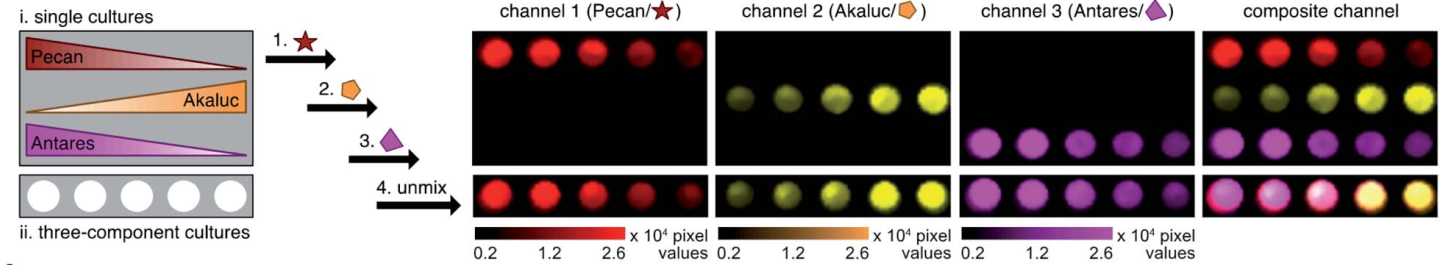
Fig. 2 Multi-component imaging with three NIR-emitting probes.
The brightest luciferase-CouLuc-1 pair exhibited higher luminescent signals compared to native bioluminescent probes and can be immediately adopted for biological imaging. Multiplexed NIR imaging could also be attained using three different analogues of the newly prepared luciferins (Fig 2). In a broader sense, synthesis of novel luminophores from simple fluorophores pave a step forward in the bioluminescent imaging field.
For details: please visit https://pubs.rsc.org/en/content/articlelanding/2021/sc/d1sc03114g
About the blogger:
Dr. Damayanti Bagchi is a postdoctoral researcher in Irene Chen’s lab at University of California, Los Angeles, United States. She has obtained her PhD in Physical Chemistry from Satyendra Nath Bose National Centre for Basic Sciences, India. Her research is focused on spectroscopic studies of nano-biomaterials. She is interested in exploring light enabled therapeutics. She enjoys travelling and experimenting with various cuisines.
You can find her on Twitter at @DamayantiBagchi.