In this Chemical Science Edge Article, the Anderson group and colleagues at the Universitiy of Oxford describe ultra-fast light harvesting materials which function in a similar way to various natural light harvesters, like, for example, those found in the chlorophyll assemblies of purple bacteria. These materials represent excellent candidates for use in next generation carbon based solar cells.
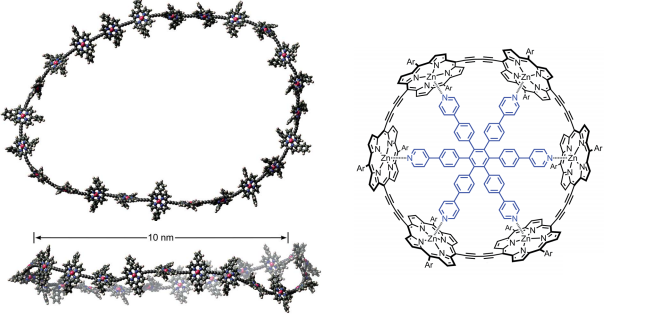
The materials, which may contain up to 24 porphyrin units separated by conjugating butadiyne bridges, can measure up to 10nm in diameter. Recent advances in template directed synthesis mean these molecules have become more accessible.
Barriers to energy delocalisation are overcome due to distortions that occur in the molecular structure. A rigidifying template was used to probe the effect of distortions – without a coordinating constraint present, significantly different behaviour was observed, underlying the importance of flexibility to the behaviour observed.
Physical techniques were used to characterise the complex phenomena being observed, including time resolved photoluminescence spectroscopy, using femtosecond LASERs and steady state fluorescence. Further information about electronic structure was gained by comparing spectra of the ring structures with those of linear oligomeric analogues.
The authors describe synthetic materials which show a level of light harvesting and rapid energy delocalising ability, usually only seen in natural systems. The promise of technological applications which exploit these properties will drive the study of the fundamental physics and chemistry of such fascinating systems.
Read this Chemical Science Edge Article today:
Ultrafast Delocalisation of excitation in synthetic light-harvesting nanorings
Chaw-Keong Yong, Patrick Parkinson, Dmitry V. Kondratuk, Wei-Hsin Chen, Andrew Stannard, Alex Summerfield, Johannes K. Sprafke, Melanie C. O’Sullivan, Peter H. Beton, Harry L. Anderson and Laura M. Herz.
DOI: 10.1039/C4SC02424A











 Flow reactors are edging towards self-regulation
Flow reactors are edging towards self-regulation

 University chemistry students are taught that the shapes and electronics of inorganic complexes are predictable. For example, d8 square-planar Pd(ii) and Pt(ii) complexes are invariably low spin, while d3–d7 tetrahedral complexes are high spin. Now, researchers in the US have thrown away the textbook by synthesising
University chemistry students are taught that the shapes and electronics of inorganic complexes are predictable. For example, d8 square-planar Pd(ii) and Pt(ii) complexes are invariably low spin, while d3–d7 tetrahedral complexes are high spin. Now, researchers in the US have thrown away the textbook by synthesising 


 Scientists in the US have developed
Scientists in the US have developed