Bacteria living on African ants make polyketides that are active against some drug resistant bacteria, new research shows.
An impending crisis due to the rise of antibiotic resistant bacteria means there is high demand for new drugs to treat infections. Natural products shape the backbone of the antibiotics we use today, over half of which derive from compounds made byStreptomyces and other soil microbes. But researchers are now looking in more unusual locations for the next generation of antibiotics.

Source: © Royal Society of Chemistry
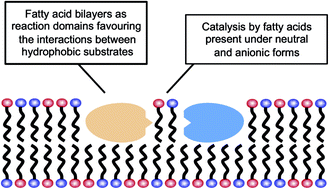
Formicamycins are more potent than the previously reported and structurally related fasamycins
Matt Hutchings from the University of East Anglia and colleagues have discovered a new family of antibacterial polyketides, called formicamycins, in bacteria living onTetraponera penzigi, a species of fungus-growing plant-ant. Not only have the team found a new family of molecules but the bacteria that made them, Streptomyces formicae, is new to the scientific community too. ‘Plant roots have lots of Streptomycesbacteria in them, and lots of insects like ants, particularly fungus-growing ants, also pick up these bacteria,’ Hutchings explains.
Read the full story by Adrian Robinson in Chemistry World.
This article is Open Access.
Z Qin et al., Chem. Sci., 2017, DOI: 10.1039/c6sc04265a












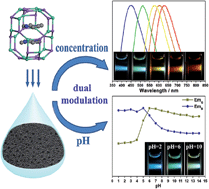
 Inspired by foams in everyday products such as food, researchers in the UK have developed a way to form extremely stable temperature-sensitive air-in-oil foams.
Inspired by foams in everyday products such as food, researchers in the UK have developed a way to form extremely stable temperature-sensitive air-in-oil foams.