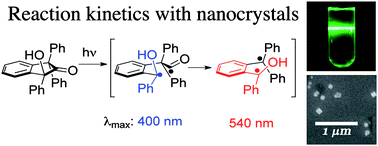
UK scientists have reported a novel, more general method for inserting a useful ‘tag’ into proteins, enabling easier protein modification.
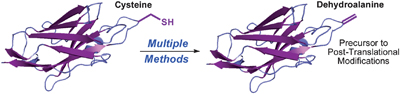 Dehydroalanine is an amino acid residue and useful precursor to a range of post-translational modifications. Several chemical and biochemical methods for incorporating dehydroalanine into peptides and proteins have been reported but each strategy has its limitations, says Ben Davis, from the University of Oxford.
Dehydroalanine is an amino acid residue and useful precursor to a range of post-translational modifications. Several chemical and biochemical methods for incorporating dehydroalanine into peptides and proteins have been reported but each strategy has its limitations, says Ben Davis, from the University of Oxford.
Davis’ team assessed the merits and drawbacks of these methods and came up with a more general method – the bis-alkylation-elimination of cysteine to dehydroalanine using a stable, easy to prepare dibromide compound.
To demonstrate the scope and utility of the method, they used it to incorporate the tag into an antibody, then glycosylated it.
To find out more about Professor Davis’ research, download his Chemical Science Edge article. You can also see him speak at Challenges in Chemical Biology (ISACS5) in Manchester in July – registration deadline 24th June 2011.











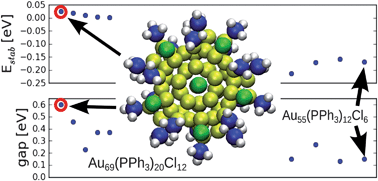 Teams from Germany, the US and Finland have re-investigated the structure of a famous gold-phosphine-halide compound.
Teams from Germany, the US and Finland have re-investigated the structure of a famous gold-phosphine-halide compound.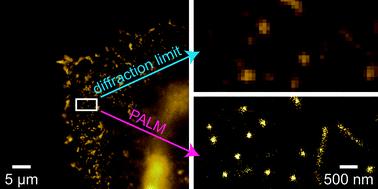 They visualised clustering of lipid molecules in nanodomains with a spatial resolution one order of magnitude better than the diffraction limit and were able to resolve the detailed structure of the domains.
They visualised clustering of lipid molecules in nanodomains with a spatial resolution one order of magnitude better than the diffraction limit and were able to resolve the detailed structure of the domains.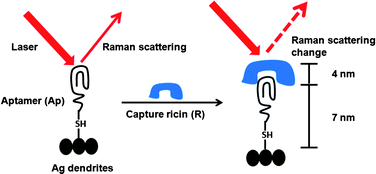 Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.
Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a ‘select’ bioterror agent and was involved in a recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.