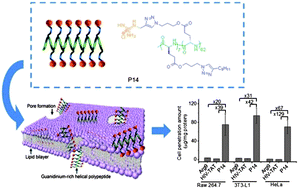
Helical poly(arginine) mimics with superior cell-penetrating and molecular transporting properties
Haoyu Tang, Lichen Yin, Kyung Hoon Kim and Jianjun Cheng
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC51328A, Edge Article
Free to access until 29th September 2013
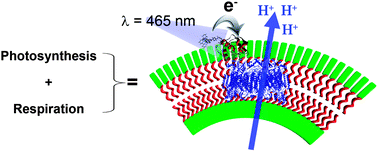
Self-assembled light-driven photosynthetic-respiratory electron transport chain hybrid proton pump
David Hvasanov, Joshua R. Peterson and Pall Thordarson
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC51780B, Edge Article
Free to access until 29th September 2013
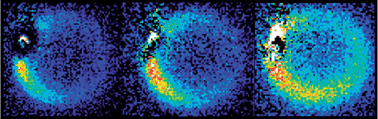
3D optical imaging of multiple SERS nanotags in cells
Sarah McAughtrie, Katherine Lau, Karen Faulds and Duncan Graham
Chem. Sci., 2013,4, 3566-3572
DOI: 10.1039/C3SC51437D, Edge Article
Free to access until 29th September 2013
Rotationally inelastic scattering of CD3 and CH3 with He: comparison of velocity map-imaging data with quantum scattering calculations
Ondřej Tkáč, Alan G. Sage, Stuart J. Greaves, Andrew J. Orr-Ewing, Paul J. Dagdigian, Qianli Ma and Millard H. Alexander
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC52002A, Edge Article
Free to access until 29th September 2013
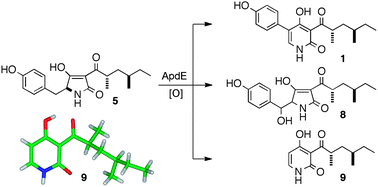
One pathway, many compounds: heterologous expression of a fungal biosynthetic pathway reveals its intrinsic potential for diversity
Zahida Wasil, Khomaizon A. K. Pahirulzaman, Craig Butts, Thomas J. Simpson, Colin M. Lazarus and Russell J. Cox
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC51785C, Edge Article
Free to access until 29th September 2013
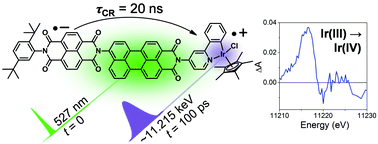
Interrogating the photogenerated Ir(IV) state of a water oxidation catalyst using ultrafast optical and X-ray absorption spectroscopy
Michael T. Vagnini, Michael W. Mara, Michael R. Harpham, Jier Huang, Megan L. Shelby, Lin X. Chen and Michael R. Wasielewski
Chem. Sci., 2013, Advance Article
DOI: 10.1039/C3SC51511G, Edge Article
Free to access until 29th September 2013

















 Scientists from the UK and Germany have developed
Scientists from the UK and Germany have developed