US scientists have come up with a method that makes it easier to extract compounds that are difficult to isolate from crude natural product mixtures.
Erin Carlson and her team at the University of Indiana, Bloomington, used resins to target and isolate desired compounds in crude extract mixtures, in this case alcohols.
Isolating compounds from natural product mixtures is important because of their high propensity to interact with biological targets. Nearly half of currently available drugs are from natural products and pharmaceutical companies analyse crude extracts from, for example, plant materials for biological activity. Any active compounds are purified either by extraction and/or chromatography.
Current extraction methods rely on the compounds’ physical properties such as solubility, polarity or size. Despite advances in separation technology, purification is still problematic and time-consuming. ‘Isolation of trace quantities of new natural products is often thwarted by the crude extract containing a plethora of compounds, with a heavy reliance on multiple liquid chromatography processes,’ says Gordon Florence, an expert in bioactive natural products from the University of St Andrews, UK.
Carlson’s team used a silyl-functionalised resin to capture and bind to the alcohol anisomycin – a protein biosynthesis inhibitor – from a mixture of compounds extracted from soil bacteria Streptomyces griseolus. The team washed the resin to remove non-targeted compounds before cleaving the anisomycin from the resin and found that the resin didn’t bind to compounds with functional groups other than alcohol. Following regeneration, the resin could be reused.
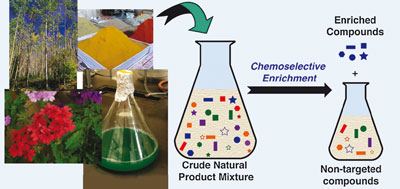
A silyl-functionalised resin was used to capture and bind to the alcohol anisomycin – a protein biosynthesis inhibitor – from a mixture of compounds extracted from soil bacteria Streptomyces griseolus
Read the full Chemistry World article here
Link to journal Article
Chemoselective enrichment for natural products discovery
Antoinette Y. Odendaal, Darci J. Trader and Erin E. Carlson,
Chem. Sci., 2011, DOI: 10.1039/c0sc00620c










