UK chemists have discovered a surprising application of a simple uranium complex that is widely used as a starting material in coordination chemistry.
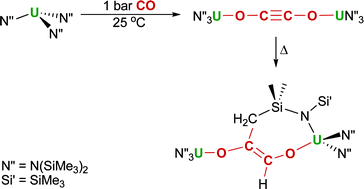
Polly Arnold, at the University of Edinburgh, and colleagues found that the uranium tris(amide) complex UN’’3 (N’’ = N(SiMe3)2) reacts with carbon monoxide gas at room temperature and pressure to give a reductively coupled [OCCO]2- fragment as the sole product.
Remarkably, as the fragment is sterically protected, it was still active towards another C–C bond forming event: upon warming it reacted with one of the amido ligand C-H groups to give a functionalised enediolate dianion.
Find out more about this fascinating reaction in Professor Arnold’s Chemical Science Edge article.