A reaction in which one of the products speeds up further product formation is called an autocatalytic reaction. Autocatalysis plays an important role in living systems including DNA replication, apoptosis, and even in the origin of life, due to self-sustaining growth and oscillation. Researchers from Brown University employ this nature of autocatalytic click chemistry to generate an artificial neural network that can be used for image classification.
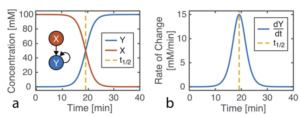
Autocatalytic reaction rate depends on the concentration of product and shows a non-linear dependency of product formation with progress in reaction time. In this view, a network of autocatalytic reactions is analogous to an artificial neural network. An artificial neuron is a basic learning unit, inspired by biological neurons, which multiplies it’s inputs by a set of weights and transforms their sum through a nonlinear operator. Researchers used this resemblance to formulate a winner-take-all neural network.
Fig 1: Kinetics of autocatalysis. (a) Reagent and autocatalytic product evolution over time (b) Rate of product concentration change over time for the reaction simulated in a, showing the accelerated production typical of an autocatalytic process.
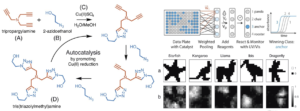
Copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction was chosen for autocatalysis as it is fast, can occur under mild conditions and produce high yield. Also, CuAAC reaction involves colored copper–ligand complexes and can be quantitatively monitored using UV-vis spectroscopy.
In a winner-take-all neural network, winner is determined by it’s achievement to reach to a particular condition. Here, they have used the reaction half-way point (t1/2) as the condition of image classification. Experiment wise, they have used automated liquid handling equipment to remove a certain volume and then added it together into individual pools for potential image class. The pool that reaches the transition time first is determined as the winner.
Fig 2: An overview of the copper (C) catalyzed azide–alkyne cycloaddition reaction, showing the buildup of triazole branches on the amine backbone of (A) after each azide (B) incorporation. The threebranched product (D) catalyzes its own generation by promoting the reduction of Cu(II). Experimental setup for evaluating a chemical WTA network (Right: upper panel). (Right lower panel) Network training and in silico simulation. (a) Example images from each of the considered classes. (b) Trained weights for each class.
This study shows an interesting adaptation of autocatalysis as a platform for non-linear activation function necessary for artificial neural network classification. The findings are expected to improve future development of chemical-domain computing systems.
For further details, please go through:
Leveraging autocatalytic reactions for chemical domain image classification
Christopher E. Arcadia, Amanda Dombroski, Kady Oakley, Shui Ling Chen, Hokchhay Tann, Christopher Rose, Eunsuk Kim, Sherief Reda, Brenda M. Rubensteinb and Jacob K. Rosenstein*
About the blogger
Dr Damayanti Bagchi is a postdoctoral researcher in Irene Chen’s lab at University of California, Los Angeles, United States. She has obtained her PhD in Physical Chemistry from Satyendra Nath Bose National Centre for Basic Sciences, India. Her research is focused on spectroscopic studies of nano-biomaterials. She is interested in exploring light enabled therapeutics. She enjoys travelling and experimenting with various cuisines, which she found resembles with products/ side products of chemical reactions!
You can find her on Twitter at @DamayantiBagchi.