Methylene (-CH2) is one of the simplest and most important building blocks for chemical synthesis. Methylenation reactions add methylene groups to molecules and often proceed using transition metal methylene complexes. Titanium methylene complexes are excellent for methylenations and have been used in a variety of reactions such as olefin metathesis, polymerisations or olefination of carbonyls. Early examples of such titanium methylenation reagents include Tebbe’s reagent that can generate a terminally bound mononuclear titanium methylidene, Cp2Ti=CH2 (Figure 1a), or a methylenation reagent prepared from CH2Br2, Zn and TiCl4 (with catalytic lead), referred to as ‘CH2X2-Zn(Pb)-TiCl4’.
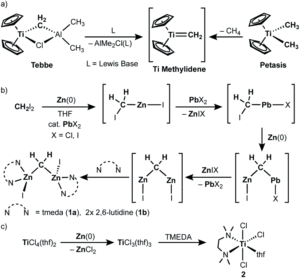
Figure 1. (a) The titanium methylidene methylenating reagent from the Tebbe or Petasis reagents. (b) The first key step for the ‘CH2X2-Zn(Pb)-TiCl4’ methylenating reagent: generation of the zinc methylene. (c) The second key step for ‘CH2X2-Zn(Pb)-TiCl4’ methylenating reagent: reduction of Ti(IV) to Ti(III).
Researchers from Japan have been interested in the ‘CH2X2-Zn(Pb)-TiCl4’ methylenation reagent and in particular, deducing the molecular structure of the reactive species. Earlier studies have revealed two key steps in the preparation of this methylenating reagent: the first is that a zinc methylene species, ‘CH2(ZnX2)’, is formed by the reaction of CH2X2 with Zn and catalytic lead (Figure 1b), and the second is that the Ti(IV) chloride reagent is reduced to Ti(III) chloride by Zn(0) simultaneously (Figure 1c). The researchers hypothesised that a reactive titanium methylidene species (similar to that generated from Tebbe’s reagent in Figure 1a) should form via a transmetallation event between the zinc methylene species and the Ti(III) chloride, and thus be the reactive methylenating species of the ‘CH2X2-Zn(Pb)-TiCl4’ methylenation reagent.
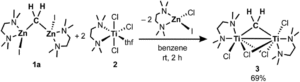
To confirm their hypothesis, the researchers studied the reactivity of multiple combinations of a zinc methylene species (1) and titanium(III or IV) chloride reagents, with and without additional ligands (such as phosphines, amines or ethers). The researchers found that most combinations of reagents resulted in methylene loss via the generation of methane or ethylene, but the combination of TMEDA adducts of the zinc methylene (1a) and Ti(III) chloride (2) gave clean conversion to a new titanium methylene species 3 (Scheme 1). Although the researchers originally hypothesised the formation of a mononuclear titanium methylidene via methylene transmetallation from zinc to titanium, the new species 3 was revealed to be a dinuclear, bridging methylene complex. The dinuclear species was characterised using NMR spectroscopy and single-crystal X-ray diffraction techniques, and the connectivity of the bridging methylene was conclusively established by the X-ray crystal structure.
After elucidating the structure of the dinuclear titanium methylene complex, the researchers tested 3 as a methylenating reagent and observed successful methylene transfer reactions from 3 to esters, terminal olefins and 1,3-dienes. A further computational mechanistic study for the reactivity of 3 and a 1,3-diene was performed, where the DFT calculations indicated a mononuclear titanium methylidene as the reactive species, generated from the dinuclear titanium methylene complex. These calculations corroborate the researchers’ initial hypothesis and correlate with Tebbe’s reagent, where the reactive methylenating agent is also a mononuclear titanium methylidene that is generated from a dinuclear bridging methylene complex.
To find out more, please read:
Takashi Kurogi,* Kaito Kuroki, Shunsuke Moritani and Kazuhiko Takai*
Chem. Sci., 2021, Advance Article
About the blogger:
 Dr. Samantha Apps recently finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps recently finished her post as a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.