Finding new ways to make chemical bonds is not only a fascination for chemists, but also important for developing greener routes in chemical synthesis. Carbon-nitrogen bonds are one such motif of interest; they are ubiquitous in chemical synthesis as part of amine functional groups, which are present in biologically relevant molecules and pharmaceutical compounds. A powerful method for the formation of C-N bonds involves using nitrenes (R-N:) as the nitrogen source. The use of azides (RN3) to generate the nitrene is particularly attractive, as the only by-product is nitrogen gas, and no additional oxidants are required.
Typical drawbacks associated with the use of nitrenes in amination reactions, i.e. forming C-N bonds for amine functionalities, include the necessity for elevated temperatures (to liberate nitrogen gas from the azide to form the nitrene) and competitive side reactivity (since nitrenes are highly reactive intermediates). Research by Che and co-workers in Hong Kong and China now describes a way to circumvent these disadvantages, using visible light in combination with an iron porphyrin complex to catalyse C-N bond formation by either C-H bond amination or alkene aziridination using organic azide substrates.
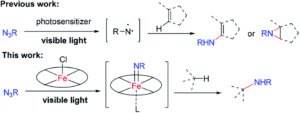
Figure 1: Trapping of the nitrene (NR) generated from an azide (N3R) by the iron porphyrin (bottom), compared to previous work (top) where a free, reactive nitrene is generated.
The iron porphyrin complex, as described by the researchers, has a dual role in the light-driven C-N bond formation reactivity. Firstly, the iron porphyrin acts as a photosensitiser in this reaction, assisting with the nitrene formation from the organic azide by irradiation. More importantly, the porphyrin complex then acts as a trap for the reactive nitrene and forms a resulting metal-nitrene (or imido) intermediate that can then react with carbon-based substrates for C-N bond formation. Capturing the nitrene at a metal centre, as opposed to generating a free nitrene for subsequent reactivity (as shown in Figure 1), allows for greater selectivity, which is reflected in the extensive substrate scope described by Che and co-workers.
The researchers started their investigation by screening a panel of iron porphyrins to serve as a catalyst in the light-driven C-H amination of indane (as a hydrocarbon substrate) with an electron-deficient aryl azide (Scheme 1). They observed the successful conversion to the C-H amination product, 1-aminoindane, in various yields, where the Fe(TF4DMAP)Cl porphyrin complex (as shown in Scheme 1) was the most effective catalyst, resulting in a 99% yield after 24 hours. Control studies confirmed both light irradiation and the iron porphyrin were required for conversion, and further mechanistic experiments supported the formation of an iron-nitrene intermediate, that could subsequently react with a C-H bond (in this case, within indane) via H-atom abstraction for amination. Translating the same reaction conditions to sp2 carbon substrates, by using styrene instead of indane, resulted in olefin aziridination, showing the applicability of this method to other substrates.
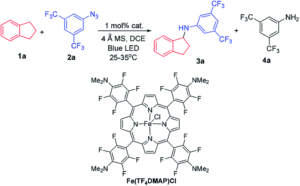
Scheme 1: C-H amination of indane (1a) with the electron-deficient aryl azide (2a) to form 1-aminoindane (3a), using the optimal iron porphyrin catalyst Fe(TF4DMAP)Cl
After determining the optimal conditions in the above example reactions, Che and co-workers demonstrated an impressive substrate scope for this reactivity, varying either the hydrocarbon substrate or the organic azide for C-H amination. Additionally, they also demonstrated this reactivity could be applied to intramolecular C-H amination, for the formation of imidazolidines or α-azidoketones. Ultimately, this reactivity could be translated to natural product synthesis, and preliminary results in this report showed that late-stage C-H amination using azides could be achieved in complex substrates.
This work elegantly demonstrates a new method for C-N bond formation using organic azides and hydrocarbon or olefin substrates, as the first example of a light driven, iron-porphyrin catalysed, C-H amination or alkene aziridination reaction.
To find out more, please read:
Yi-Dan Du, Cong-Ying Zhou, Wai-Pong To, Hai-Xu Wang and Chi-Ming Che
Chem. Sci., 2020,11, 4680-4686










