Nature is the ultimate molecular designer. The complex structures of proteins are crucial for allowing the many processes that support life, including the many chemical transformations that occur. A diverse array of biological reactions are catalysed by iron porphyrin active sites (also known as hemes), and rely on the local protein environments to envelop and stabilise the reactive intermediates that can form in the process. Whilst chemists can easily synthesise iron porphyrins to imitate the reactive centre, mimicking the surrounding protein superstructure is less trivial.
Metal-organic frameworks (MOFs) represent one strategy (as an alternative to proteins) to support iron porphyrins for chemical catalysis. These frameworks can precisely separate each heme unit, thereby sequestering each active site in a similar fashion to a protein, and the porous nature allows for diffusion of reagents into the catalyst. Importantly, the pore environment can be precisely controlled and modified, allowing for enhancements to the catalytic activity of the supported heme.
Researchers in the US have now reported a new method to modify a heme-containing metal-organic framework to enhance the catalytic activity towards C-H bond activation. They studied the porphyrinic Zr-based framework, PCN-224, where the porphyrin is suspended between Zr6 nodes (Figure 1). Exchange of the formate and benzoate ligands around the Zr6 node in PCN-224 was achieved by initial treatment with acetic anhydride to give acetate ligands in the new material (PCN-224’, 1), and further reactivity with methanol resulted in Zr-hydroxy ligands (2) – see insert to Figure 1. Additionally, iron was installed into the modified framework 1 by reaction with FeCl3 and base, to give 1FeCl. Here, an FeCl was installed in each porphyrin unit in the MOF, and further hydroxylation reactivity (similar to the 1 to 2 transformation) resulted in the formation of 2FeCl.
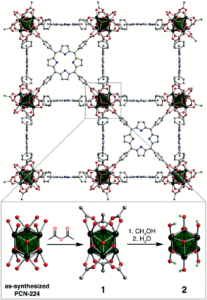
Figure 1. The structure of the PCN-224 framework. Insert (below) shows modifications to PCN-224 to give 1 and 2, with varying ligands around the Zr6 node (in green).
The modified PCN-224 frameworks were characterised by various techniques. Powder X-ray diffraction showed the retention of bulk crystallinity of the material upon ligand substitution. UV-Vis and 57Fe Mössbauer spectroscopy confirmed iron coordination within the porphyrin units of the framework. Importantly, the modification of the Zr6 ligands was structrually confirmed by single-crystal X-ray analysis, and DRIFTS spectra showed the expected O-H stretches for the hydroxy ligands in 2 and 2FeCl. The porosity of the framework was maintained upon the modifications, as shown by surface area measurements, making the new materials ideal candidates for catalytic testing.
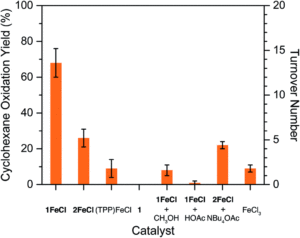
Figure 2. A chart showing the catalytic activity of the heme-frameworks (1FeCl and 2FeCl) compared to the molecular iron porphyrin complex ((TPP)FeCl) for cyclohexane oxidation.
The researchers studied the effects of the framework modifications using the catalytic oxidation of cyclohexane as a model reaction. The researchers compared the oxidation of cyclohexane with iodosylbenzene in CH2Cl2 using either the molecular iron porphyrin complex, or the metallated porphyrin frameworks 1FeCl or 2FeCl. Whilst low yields of oxidation products (cyclohexanol, cyclohexanone and chlorocyclohexane) were observed for the molecular iron porphyrin complex, higher yields of 68% and 26% were noted for the frameworks 1FeCl and 2FeCl, with corresponding turnovers of 14 and 5, respectively (Figure 2). The higher catalytic activity for the acetylated framework (1FeCl) was attributed to the lack of acidic protons within the framework that would impair any oxidation reactivity at the heme centre. Ultimately, the results show an enhancement to the catalysis when the heme is supported and protected, demonstrating that MOFs are ideal supports for modelling enzymatic reactions.
To find out more, please read:
Enhancing catalytic alkane hydroxylation by tuning the outer coordination sphere in a heme-containing metal–organic framework
David Z. Zee and T. David Harris
Chem. Sci., 2020, 11, 5447-5452
About the blogger:
 Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.
Dr. Samantha Apps is a Postdoctoral Research Associate in the Lu Lab at the University of Minnesota, USA, and obtained her PhD in 2019 from Imperial College London, UK. She has spent the last few years, both in her PhD and postdoc, researching synthetic nitrogen fixation and transition metal complexes that can activate and functionalise dinitrogen. Outside of the lab, you’ll likely find her baking at home, where her years of synthetic lab training has sparked a passion in kitchen chemistry too.










