Doesn’t everyone love gold? Not only is it shiny and pretty in macroscopic form, but it’s one of the best-behaved nanoscale systems and the focus of extensive catalysis study. While much is known about the mechanisms of many gold-catalyzed reactions, a question of whether a number of organogold complexes are actual intermediates or off-cycle sinks remains. Catalysis of the nucleophilic addition of water to alkynes via gold complexes is a reaction with multiple hypothesized active intermediates and reaction pathways. Initially thought to occur via monoaurated species, subsequent work proposed activation by multiple gold catalysts. The problem, as seen in figure 1, is that the two pathways are connected and the presence of any specific intermediates can’t rule either pathway out.
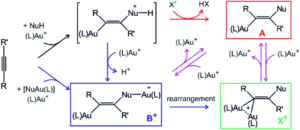
Figure 1. Reaction scheme with potential intermediates for nucleophile addition to an alkyne via a gold-catalyzed pathway.
To solve this problem, researchers in the Czech Republic and the Netherlands developed a method to probe solution-phase intermediates with electrospray ionization mass spectrometry (ESI-MS) called Delayed Reactant Labeling. To do this, one of the reactants must be a mixture of isotopically labeled and unlabeled molecules, added separately with a time delay. This helps eliminate ionization artifacts and moves the reaction away from steady state conditions to allow for kinetic modeling. Using this technique, combined with other more standard characterization methods like NMR and infrared (IR) spectroscopy, the researchers studied gold-catalyzed water addition to alkynes. The catalyst is known to form digold hydroxides in the presence of water, which lends credence to the idea that a digold species is involved in the catalysis. Based on kinetic restrictions, they studied the addition of water to 1-phenylpropyne, which produces a mixture of regioisomers of intermediates that was a bit challenging to deconvolute. The initial ESI-MS spectra show the presence of both mono- and diaurated species and the different fragments were isolated, analyzed by IR photodissociation, and the spectra compared to theoretical models to corroborate their identity.
These results set the stage for the Delayed Reactant Labeling studies using deuterated 1-phenylpropyne. After the reaction reached equilibrium, in this case about 40 minutes, the labeled reactant was added to then allow for kinetic fitting of the intermediates. They determined that under standard conditions the monoaurated species has a half life of approximately 9 minutes and the diaurated species has a half life of 7 minutes. These decay constants could be altered by adding organic acids to degrade the complexes faster, while attempts to trap the species as salts were unsuccessful. Upon reaction with D2O a kinetic isotope effect doubling the lifetimes was observed and suggests that the mechanism is likely the same for all intermediates and that it involves a hydrogen/proton transfer. The two types of species also have slightly different rates of formation, with the diaurated species likely having a higher turn-over frequency. However, there isn’t a dramatic difference between the kinetics of these two types of intermediates.
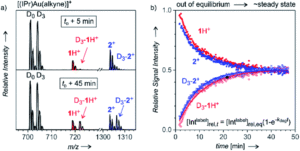
Figure 2. Example of a) spectra obtained from the delayed reactant labeling method and b) fits of peak intensities over time used to extract kinetic information.
In order to determine which of the intermediates is catalytically relevant, the researchers changed the substrate to 3-hexyne. The symmetric alkyne has no regioisomeric intermediates to convolute the data, but the reaction kinetics are much faster and therefore not suited to the prior mechanistic studies. By adding an excess of acid, the rate determining step was moved from protodeauration. Under these conditions, the rate has a linear dependence on the gold complex and likely proceeds primarily via monoaurated intermediates. This approach combining multiple analytical techniques elucidated the role of various gold-containing intermediates and demonstrated the utility of ESI-MS as a tool for determining reaction kinetics.
To find out more, please read:
Monoaurated vs. diaurated intermediates: causality or independence?
Mariarosa Anania, Lucie Jašková, Jan Zelenka, Elena Shcherbachenko, Juraj Jašk and Jana Roithová
Chem. Sci., 2020, Advance Article
About the blogger:
 Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.










