While nanobots are still a thing of science fiction, the development of the fundamental scientific concepts that could lead to highly complex molecular-scale machines has been an active area of research for over 2 decades. Molecular motors rely on isomerization through metastable intermediate states that leads to unidirectional rotation via a rotor portion of the molecule. Initial work utilized molecules that convert high-energy UV-light into this directional motion. However, these systems are limited in their future utility, particularly in biological applications, if they need UV-light for activation. Recent work focuses on driving molecular motors with visible light via a range of approaches, including altering the absorbance profile of the motor molecule itself. Unfortunately, changing the electronics of the molecule has previously substantially decreased the quantum yield while resulting in only slight red shifts.
Researchers in The Netherlands recently developed 2nd generation molecular motors featuring a mixture of electron-donating and electron-withdrawing groups that exhibit substantial red-shifts. Density functional theory (DFT) calculations predicted that adding cyano- and methoxy- groups to opposite halves of the motor would shift the absorbance past 410 nm. The new molecules were synthesized via a general procedure shown in Figure 1, with yields ranging from 3 – 10%.
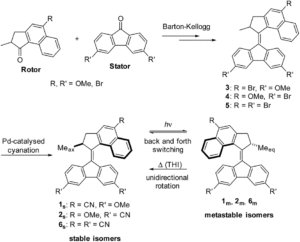
Figure 1. General synthetic route to cyano- and methoxy- substituted molecular motors and photoisomerization reaction that results in unidirectional motion.
The addition of the cyano- and methoxy- groups shifted the absorbance maxima corresponding to the HOMO-LUMO transitions to from 422 – 453 nm, an increase of over 60 nm from the parent molecule. When irradiated, the molecules transitioned from their stable ground state to the metastable isomers. These were characterized by UV-Vis spectroscopy, with the emergence of further red-shifted features (Figure 2), and 1H NMR. The 1H NMR spectra were obtained with in-situ irradiation (which sounds like a sweet experimental setup) at various wavelengths and the ratios of several specific protons on the rotor. The derivative 2 with a methoxy- group on the rotor and cyano- groups on the stator demonstrated activity with irradiation at wavelengths up to 530 nm.
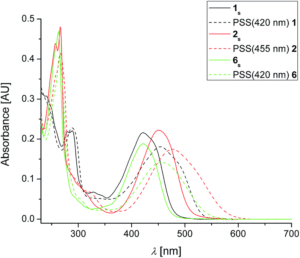
Figure 2. UV-Vis absorption spectra of stable (solid) and metastable (dashed) isomers of the molecular motors.
The energy of activation of the rotation for all three derivatives was determined by Eyring analysis and corroborated by DFT calculations. All were around 90 kJ/mol, with 2 requiring the most energy to elongate the central alkene bond and isomerize. The quantum yields of the motors for the forward reaction range from 5.8 – 11.5%, comparable to state-of-the-art UV absorbing motors. The quantum yields for the back reactions were calculated to be significantly lower than those for the forward reaction, which corresponds to the excess of metastable isomers observed under active irradiation. These motors also exhibit high photostability, with no significant change in the ground state absorbance after irradiation and cycling. This is promising for smart materials applications where stability is crucial. This work pushes forward the design of molecular motor systems that utilize visible rather than UV light.
To find out more please read:
Photoefficient 2nd generation molecular motors responsive to visible light
Lukas Pfeifer, Maximilian Scherübl, Maximilian Fellert, Wojciech Danowski, Jinling Cheng, Jasper Pol and Ben L. Feringa
Chem. Sci., 2019, 10, 8768-8773.
About the blogger:
 Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.










