As we transition to an energy future composed primarily of intermittent, renewable technologies, finding ways to store the excess generated charges will be critical. While the general public typically thinks of batteries, another area of massive interest is storing the electrons in a chemical bond. This can be accomplished by creating electrocatalysts that can couple an electric current with chemicals like water and oxygen to generate stable new bonds in molecules for later use as fuels. These renewably generated fuels would then be available whenever needed, like at night. However, finding efficient electrocatalysts composed of earth-abundant materials has proven challenging. Many researchers have turned to nature for inspiration by designing molecules that mimic the active sites of enzymes.
Researchers in the United States used this approach, focusing on creating high-valent iron-oxo species, which others previously identified as the key catalytic intermediates in multiple enzymatic reactions. These types of species have traditionally been synthesized by reacting a reduced iron complex with an oxygen transfer reagent, but the researchers developed a system to generate highly reactive species using electricity as the reaction driving force and water as the oxygen source. The studied catalyst is a commercially available iron(III)-aquo complex with a tetraamido macrocyclic ligand (TAML) as the ancillary ligand (Figure 1B).
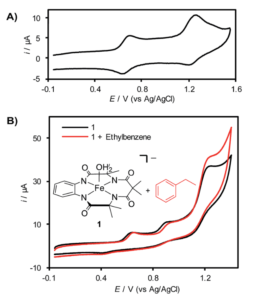
Figure 1. A. Cyclic voltammagram of (TAML)Fe in acetonitrile. B. Structure of (TAML)Fe and cyclic voltammagram showing increased current upon the addition of ethylbenzene.
When analyzed by cyclic voltammetry, an electrochemical technique where you cycle the voltage between set points and measure the current output, the (TAML)Fe shows two redox events at around 650 and 1250 mV that the researchers attributed to generating the FeIV-OH and FeV(O) species (Figure 1). Addition of ethylbenzene, which should react with the FeV species, increased the current at voltages of 1250 mV and higher, indicating (TAML)Fe turnover. However, isolating the Fev(O) species proved challenging as it reacts rapidly with the (TAML)Fe to form an FeIV dimer. This also limits the efficiency of the overall system by decreasing the amount of the most reactive species in solution.
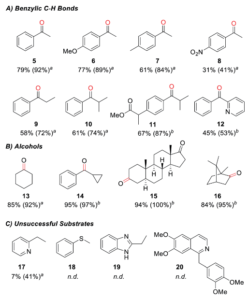
Figure 2. A, B. Products generated by oxidation of various substrates screened with (TAML)Fe for electrocatalysis with isolated yield and calculated conversion in parenthesis. C. Substrates that did not react with the (TAML)Fe complex.
Both the FeIV dimer and FeV(O) species proved capable of oxidizing C-H bonds in ethylbenzene, but the FeV(O) is much more reactive and increases the oxidation rate at high electrochemical potentials. The researchers tested the scope of (TAML)Fe reactivity using a series of compounds with benzylic C-H bonds (Figure 2). They found that the (TAML)Fe performed well with electron-rich and electron-neutral derivatives, with an electron-deficient nitro-substituted derivative showing lower reactivity. Several substrates with non-benzylic C-H bonds showed high selectivity for oxidation at the benzylic C-H bond. (TAML)Fe also showed high electrocatalytic activity for oxidizing alcohols and converted substrates as simple as cyclohexanol and as complex as a steroid to ketones in high yields (up to 97%).
This study of an earth-abundant, stable, and commercially available electrocatalyst acts as a baseline for further studies with other similar metal complexes. Despite the efficiency limits attributed to dimerization, the high stability and selectivity of the (TAML)Fe could lead to its use with a broader range of substrates with varied functional groups.
To find out more please read:
Amit Das, Jordan E. Nutting and Shannon S. Stahl
Chem. Sci., 2019, 10, 7542-7548
About the blogger:
 Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.










