Metal-organic frameworks, commonly known as MOFs, are one to three-dimensional structures composed of metal ions coordinated to organic linkers. They’ve drawn substantial research interest given their highly porous nature, the extreme tunability of their properties, and, in the early days, relative ease of synthesis. As the field has matured, the syntheses of the organic linkers have increased in complexity. New linkers require substantial expertise in synthetic organic chemistry and can be time and cost intensive to produce. One strategy to avoid the linker-induced bottleneck in MOF development is to create one-pot procedures, generating both the linker and the MOF in a single vessel. While the idea is straightforward, in practice it involves carefully balancing conditions to crystalize the MOF without producing unwanted side reactions.
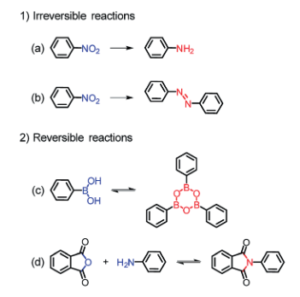
Figure 1. Reaction motifs utilized for in-situ ligand generation. a) nitro-compound reduction, b) diazo coupling of nitro compounds, c) condensation of boronic acids, and d) imidization of an anhydride and an amine.
Researchers in China recently examined several classes of organic reactions to test the viability of in-situ ligand and MOF synthesis. The basic procedure involves complex ligand generation, formation of small metal clusters, and finally crystallization of the final MOF structure. They chose reduction and diazo coupling of nitro compounds, condensation of boronic acids, and imidization between anhydrides and amines (Figure 1). A rigid, nitro-containing dicarboxylic acid proved the most robust for the reduction studies. When combined with a hydrated metal salt (copper, zinc, and indium nitrates and manganese chloride), exposed to a protic solvent, and heated, a MOF formed in a single vessel without the addition of a purposeful reductant. This specific ligand didn’t have the proper geometry to produce MOFs via diazo couplings, but a similar motif was used to create a new ligand with greater distance separating the carboxylic acid groups. The researchers dissolved the ligand and various metal salts in DMF, then added proton source, and heated the mixture. The reactions with zirconium, zinc, cadmium, and indium all produced MOFs. The reaction conditions varied from metal to metal, producing different forms of the ligand in-situ that resulted in MOFs of a range of morphologies (Figure 2).
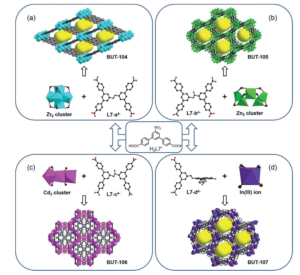
Figure 2. Structures of zirconium, zinc, cadmium, and indium-based MOFs synthesized by ligands generated via diazo coupling.
While these MOFs formed via strong, irreversible reactions, the covalent organic framework literature utilizes the plethora of reversible reactions to expand the scope of possible MOFs. This inspired the researchers to use boronic acid derivatives as a proof of concept. When a ligand with both boronic and carboxylic acid motifs reacted with zirconium or hafnium and formic acid, the researchers isolated a MOF with tetrahedral cages. This approach also proved successful when combining two ligands in a Schiff base synthesis to form a zirconium-based MOF. The reversible reactions required meticulous tuning of the acid source to effectively crystalize and assemble the MOFs. However, the ease of reaction set up allows more rapid screening of conditions than full-scale ligand synthesis.
This relatively simple and efficient strategy for producing new MOFs likely has broader applications than the few reactions currently explored. This will hopefully increase the speed of new MOF discovery with increasingly complex ligands.
To find out more please read:
Constructing new metal-organic frameworks with complicated ligands from “One-Pot” in situ reactions
Xiang-Jing Kong, Tao He, Yong-Zheng Zhang, Xue-Quian Wu, Si-Nan Wang, Ming-Ming Xu, Guang-Rui Si, and Jian-Rong Li
Chem. Sci., 2019, 10, 3949 – 3955.
About the blogger:
Beth Mundy is a PhD candidate in chemistry in the Cossairt lab at the University of Washington in Seattle, Washington. Her research focuses on developing new and better ways to synthesize nanomaterials for energy applications. She is often spotted knitting in seminars or with her nose in a good book. You can find her on Twitter at @BethMundySci.











