Sunlight-assisted water splitting represents a sustainable way to convert solar energy into chemical energy in hydrogen and oxygen gases. Due to its high activation energy, the oxygen evolution reaction (OER) requires large overpotential for initiation. Developing suitable OER catalysts to reduce the overpotential thus becomes instrumental for the feasibility of solar energy harvesting.
Recently, a group of scientists led by Rui Cao from Renmin University of China, and Shaanxi Normal University, China, has developed a water-soluble Cu(II)-porphyrin complex as a high-performance OER catalyst. This breakthrough has been published in Chemical Science (DOI: 10.1039/C8SC04529A).
Inspired by the molecular structure of a natural OER catalyst in the photosynthesis system – photosystem II (PSII), the researchers designed a Cu2+-coordination compound with a porphyrin ligand, tetrakis(4-N-methylpyridyl)porphyrin (Figure 1a), which mimics the structure of PSII. This biomimetic Cu2+-complex exhibits outstanding catalytic OER activity in a phosphate buffer solution at pH=7.0. The current of the cyclic voltammogram of the Cu2+-complex increases sharply (due to O2 evolution) at an onset potential of 1.13 V vs. normal hydrogen electrode (Figure 1b), corresponding to an OER overpotential of 310 mV. For comparison, the cyclic voltammograms of a blank buffer solution and a CuSO4-containing buffer solution show no pronounced current enhancement (Figure 1b), indicating the electrolyte itself and the un-coordinated Cu2+ cannot generate O2 within the tested potential range. The 310 mV overpotential is approximately two times smaller than the typical values exhibited by previously reported Cu complexes.
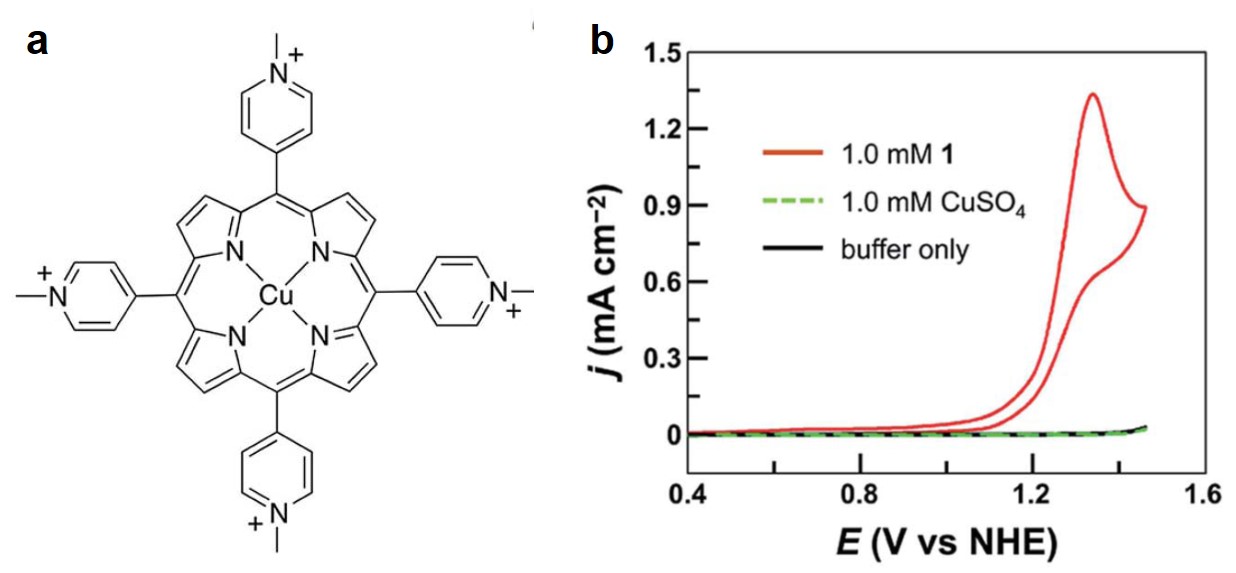
Figure 1. (a) The molecular structure of Cu2+-tetrakis(4-N-methylpyridyl)porphyrin complex. (b) Cyclic voltammograms of 1 mM Cu2+-tetrakis(4-N-methylpyridyl)porphyrin (red), bare buffer solution (black) and buffer solution containing 1 mM CuSO4 (green). The electrode is a piece of fluorine-doped tin oxide glass slide.
The authors ascribed the ultra-small OER overpotential to the formation of an oxidized form of the Cu2+-porphyrin complex. This oxidized species is generated after the complex loses one electron, and is active for O-O bond formation and subsequent O2 evolution. The energy barrier of this one-electron-oxidation pathway is expected to be much lower than those of conventional processes involving higher-valent Cu species (e.g., Cu4+-oxo), which facilitates OER at small overpotential.
With the complete catalytic cycle of water oxidation by the Cu2+-porphyrin complex being fully revealed, OER will become more efficient and energy-saving.
To find out more please read:
Low Overpotential Water Oxidation at Neutral pH Catalyzed by A Copper(II) Porphyrin
Yanju Liu, Yongzhen Han, Zongyao Zhang, Wei Zhang, Wenzhen Lai, Yong Wang and Rui Cao
Chem. Sci., 2019, DOI: 10.1039/C8SC04529A
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










