Yu and coworkers from The University of Queensland, Australia have introduced an aluminum-selenium (Al-Se) battery as a new member of the rechargeable Al-ion battery family. This battery reported in Chemical Science exhibited a capacity of 178 mAh per gram of Se, high discharging voltage above 1.5 V and satisfactory lifetime.
Al-ion batteries have attracted increasing attention as next-generation energy-storage devices. They are potentially more affordable and safer than Li-ion batteries, due to the natural abundance and the existence of native oxide surface layers of aluminum, respectively. One of the major challenges hindering the wide application of Al-ion batteries is the lack of feasible cathode materials. Previously investigated cathodes have drawbacks of low charge-storage capacity, low discharging voltage, poor electrical conductivity or chemical instability.
Inspired by sulfur, Yu and coworkers selected selenium as a cathode material for Al-ion batteries. Selenium has substantially higher electrical conductivity and lower ionization potential than sulfur, which is expected to improve the energy-storage capacity of batteries. However, a major drawback of selenium is that the oxidation product generated upon charging batteries, Se2Cl2, can dissolve quickly in electrolytes and lead to battery failure. Solving this problem would make selenium a promising cathode for Al-ion batteries.
To resolve this issue, the authors introduced a mesoporous carbon named CMK-3, nanorods that are capable of physically adsorbing Se2Cl2. The cathode, composed of Se nanowires and CMK-3 nanoparticles, is thus anticipated to improve the lifespan of batteries, as any Se2Cl2 that is generated will be confined inside the pores of CMK-3 (Figure 1).
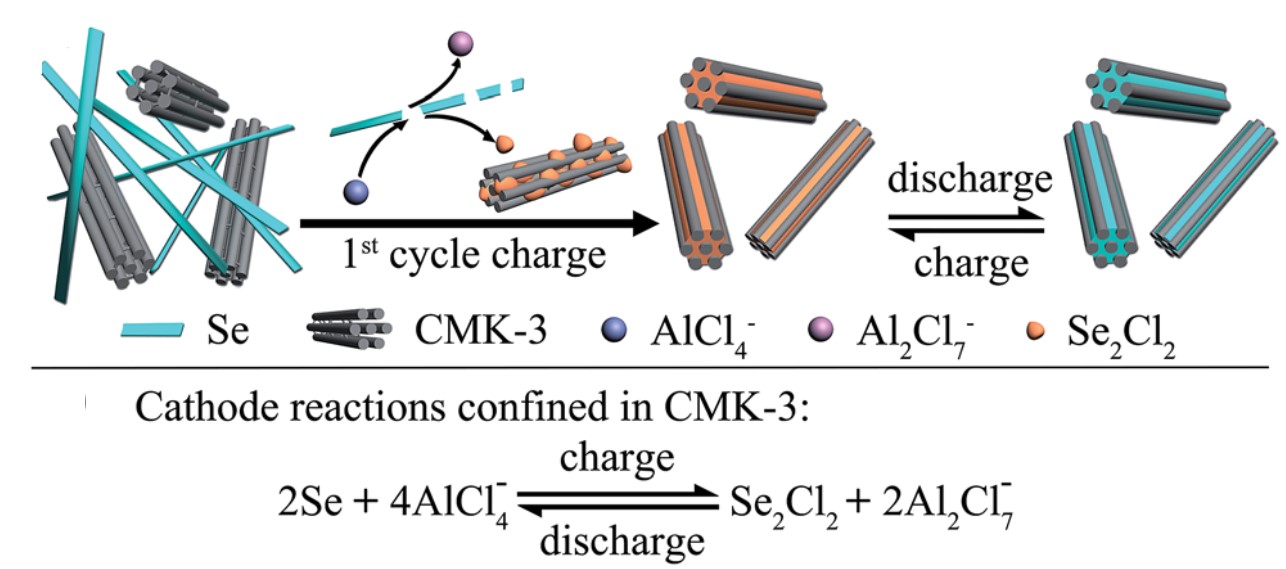
Figure 1. A schematic illustrating the CMK-3’s capability of trapping Se2Cl2. The chemical equation below shows how selenium reacts with aluminum during charge and discharge processes.
As expected, the performance of these Al-Se batteries was stable. They retained more than 80% of the initial capacity after 50 consecutive charge-discharge cycles at 100 mA/g (Figure 2a). Additionally, the discharging capacity of the batteries reached 178 mAh per gram of selenium at 100 mA/g, and the discharging potential was above 1.5 V (Figure 2b).
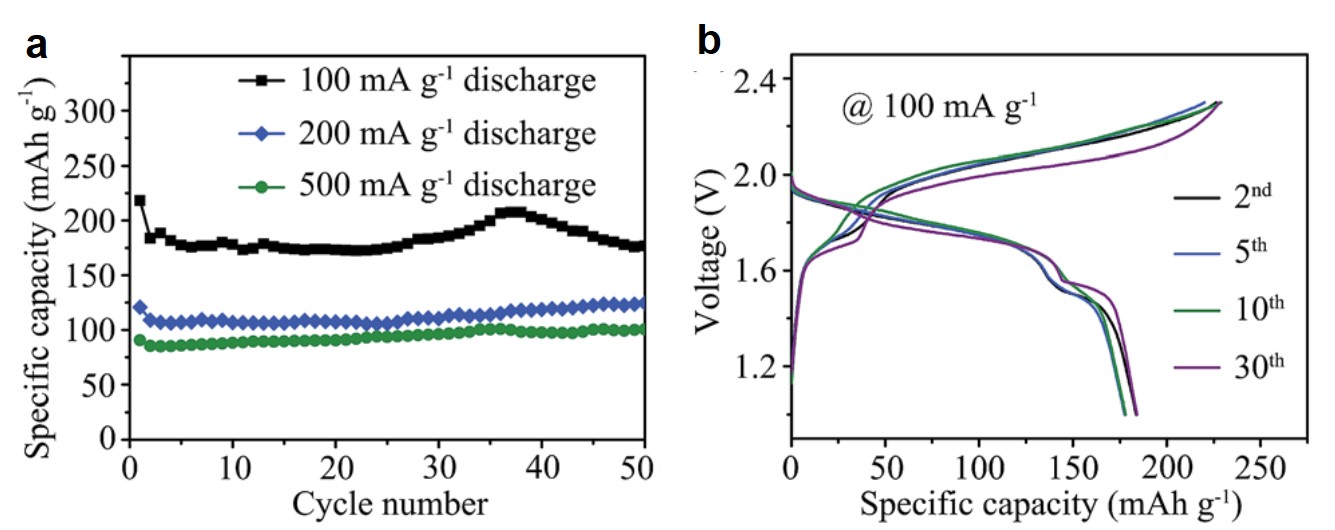
Figure 2. (a) The specific capacity of the Al-Se batteries of each cycle at different current densities. (b) The variation of battery potential with specific capacity of the 2nd, 5th, 10th, and 30th charge-discharge cycles.
These promising Al-Se batteries could encourage future work to continue progress into the development of affordable and durable Al-ion batteries.
To find out more please read:
Rechargeable Aluminum-Selenium Batteries with High Capacity
Xiaodan Huang, Yang Liu, Chao Liu, Jun Zhang, Owen Noonan and Chengzhong Yu
Chem. Sci., 2018, DOI: 10.1039/C8SC01054D
About the blogger:
 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in the United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is a blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at http://liutianyuresearch.weebly.com/.










