Biological systems, such as circadian rhythms and ion pumps, are generally able to respond autonomously to chemical stimuli. Such ability is at the root of certain amphiphilic molecules (i.e. molecules containing both hydrophilic and hydrophobic parts) that can change their configurations temporally at the presence of the stimulants. In recent decades, building artificial biological systems with active, adaptive and autonomous behaviours similar to natural biological systems has become a hot topic.
Supramolecular assemblies are among the most extensively explored building materials. A supramolecular assembly is a system consisting of complex molecules held together by non-covalent bonds, such as DNA. One of the major technological hurdles for developing artificial biological systems using supramolecular assemblies is to acquire assemblies with an amphiphilic nature and the ability to undergo a transient change of configuration when stimulated. The change should also be highly reversible and durable. Currently, extensive research efforts are committed to finding candidates that address the aforementioned challenge.
A research group from Jawaharlal Nehru Centre for Advanced Scientific Research in India recently made a step forward and published their work in Chemical Science. They developed an amphiphilic supramolecular motif, coined PN-VN foldamer, which could switch its conformation upon contact with oxidizing and reducing agents. As shown in Figure 1a, the skeleton of the supramolecular motif is composed of three sections. The green head is an electron donor pyranine (PN), the red tail is an electron acceptor called viologen (VN) rendering the hydrophobic nature, and the blue body is a flexible hydrophilic hexaethylene glycol that connects the electron donor and acceptor.
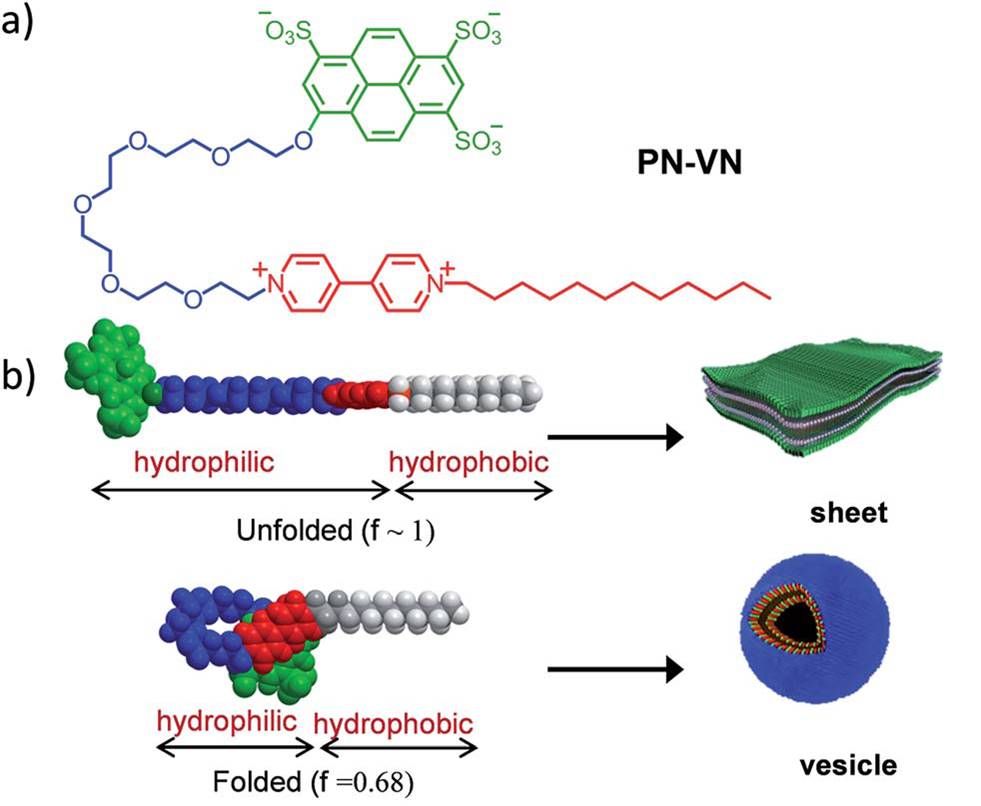
Figure 1. (a) The structure of the amphiphilic PN-VN foldamer. (b) Unfolded and folded states of the PN-VN foldamer correspond to sheet and vesicle morphologies, respectively.
The researchers demonstrated the assembly pattern of PN-VN foldamers (Figure 1b) by using two chemical fuels, sodium dithionite and glucose, as the stimulants. The transformation process is depicted in Figure 2a. When undisturbed, the negatively charged PN (PN3-) and the positively charged VN (VN2+) can attract each other via a charge transfer interaction, folding the entire molecular chains to vesicles. When placed in a solution containing sodium dithionite and glucose, the VN2+ terminal can be instantaneously reduced by sodium dithionite to its radical cation form (VN•+). The reduction weakens the charge transfer interaction, and subsequently unfolds PN-VN vesicles to sheets. Meanwhile, catalyzed by glucose oxidase (an enzyme), the excess glucose in the same solution can oxidize VN•+ back to its original state, resulting in the folded state that recovers vesicles. The transition is directly confirmed by transmission electron microscopy as shown in Figure 2b. Since the reduction process proceeds much faster than the oxidation process, it is observed that PN-VN vesicles first extend to sheets momentarily and gradually but automatically fold back to vesicles within minutes. The rate of the transition can be well tuned by varying the concentration of glucose oxidase.

Figure 2. (a) Schematic of the transient configuration change between vesicles and sheets. SDT: sodium dithionite; GOx: glucose oxidase. (b) Transmission electron microscopic images of the vesicles (left) and the sheets (right). Inset in the left panel shows the distribution of the wall thickness of the vesicles. Inset in the right panel is a confocal fluorescence microscopy image of a selected sheet (scale bar: 2 μm).
To find out more please read:
Temporal Switching of an Amphiphilic Self-assembly By a Chemical Fuel-driven Conformational Response
Krishnendu Jalani, Shikha Dhiman, Ankit Jain, and Subi J. George
DOI: 10.1039/c7sc01730h
About the author:
 Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.
Tianyu Liu is a Ph.D. in chemistry graduated from University of California-Santa Cruz. He is passionate about scientific communication to introduce cutting-edge researches to both the general public and the scientists with diverse research expertise. He is a web writer for the Chem. Commun. and Chem. Sci. blog websites. More information about him can be found at http://liutianyuresearch.weebly.com/.










