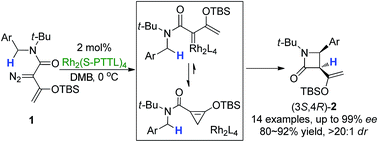
Although substantial advancements have been made in the functionalization of C–H bonds of diazo compounds, accessing β-lactams via intramolecular pathways remains a challenge. Researchers must address the chemo-, regio-, and stereoselectivity of diazoacetamide derivatives based on their electronic, steric, and conformational impacts on β-lactam synthesis
In this recent Chemical Science Edge Article, Professor Michael P. Doyle and his coworkers report a very selective asymmetric intramolecular C–H functionalization reaction of enoldiazoacetamides catalyzed by a chiral dirhodium carboxylate to achieve cis-β-lactam scaffolds.
The broad-spectrum biological activities of β-lactam motifs in treatment have proven valuable since the discovery and development of penicillin antibiotics. The magnitude of the joint scientific achievement of Sir Alexander Fleming, Ernst B. Chain and Sir Howard W. Florey in penicillin research were recognized by their receipt of the Nobel Prize in Physiology or Medicine in 1945.
The Doyle group prepared a number of donor–acceptor enoldiazoacetamides to extend beyond the less selective acceptor–acceptor diazoamides. By capitalizing on a sterically hindered dirhodium carboxylate catalyst, donor-acceptor cyclopropene intermediates led to the key intramolecular C–H insertion. Exclusive formation of cis-β-lactams resulted in high yields (80-92%) and notably high enantioselectivities (83-99% ee).
I recommend reading “Enantioselective cis-β-lactam synthesis by intramolecular C–H functionalization from enoldiazoacetamides and derivative donor–acceptor cyclopropenes” by Professor Michael P. Doyle and his colleagues to learn more about their asymmetric approach to β-lactams*.
Enantioselective cis-β-lactam synthesis by intramolecular C–H functionalization from enoldiazoacetamides and derivative donor–acceptor cyclopropenes
Xinfang Xu, Yongming Deng, David N. Yim, Peter Y. Zavalij and Michael P. Doyle �
Chem. Sci., 2015, Advance Article
DOI: 10.1039/C4SC03991B, Edge Article
*Access is free through a registered RSC account
Dr. Tezcan Guney is a guest web writer for Chemical Science. Dr. Guney received his Ph.D. from the Department of Chemistry at Iowa State University with Prof. George Kraus, where he focused on the synthesis of biologically active polycyclic natural products and multifunctional imaging probes. Currently, he is a postdoctoral research scholar at the Memorial Sloan-Kettering Cancer Center in New York with Prof. Derek Tan, contributing to the efforts to access biologically active small molecules using the diversity-oriented synthetic approach.