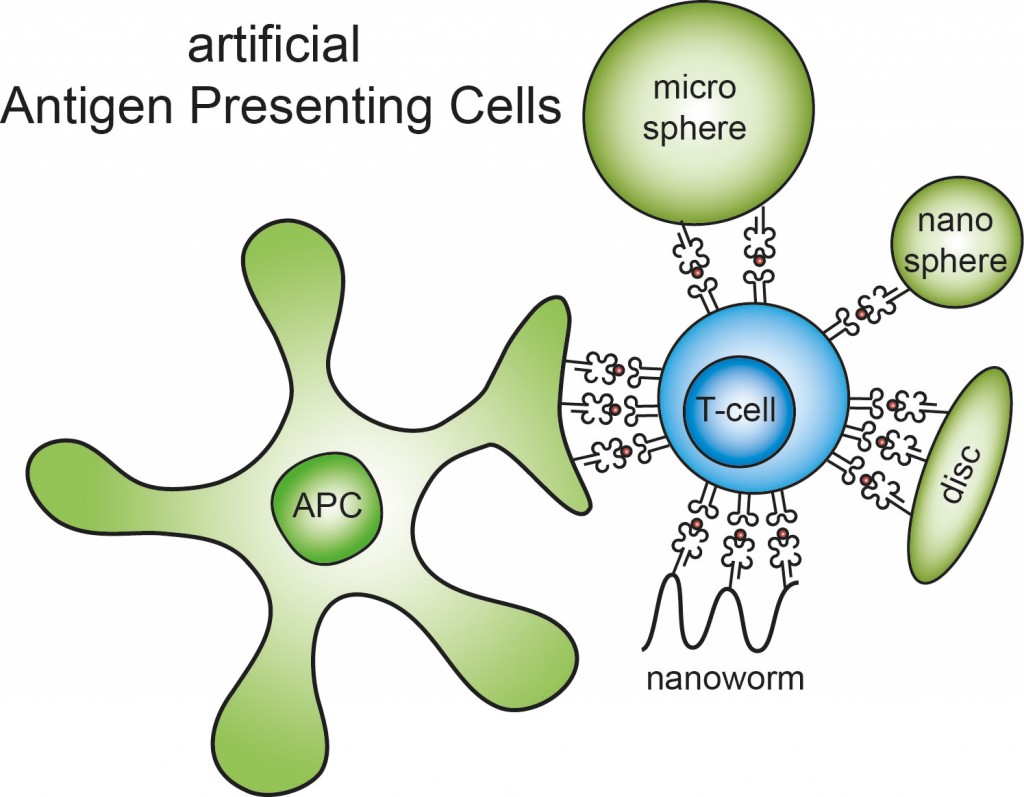
Antigen-presenting cells (ACPs) are key players in the immune systems fight against cancer. On detection of tumor tissues, these ACPs pick up antigenic fragments of the tumor cell, migrate to the lymphoid organs and present the antigen to T-cells which play a central role in cell mediated immunity. Therefore, artificial ACPs (aAPCs) are often used in the development of immune-mediated anti-cancer therapies as they show great potential to mimic antigen-presentation, promoting T-cell activation.
Carl Figdor, a world-class immunologist, and colleagues from Radbound University discuss the current status of aAPC development in a Chemical Science Perspective. This review is partly focused on the developments in nanoscience which might improve future designs for immune-mediated anti-cancer therapies.
As a synthetic mimic, aAPCs must encompass the three signals that natural APCs use to encourage T-cell activation. This perspective discusses how these signals have been incorporated into aAPC designs, but also how physical properties such a size and shape are essential for targeting the aAPCs to T-cell rich areas in vivo.
Artificial antigen-presenting cells have the potential to develop into a widespread and powerful therapeutic tool. To download the full perspective, click on the link below – access is free for a limited time only!