Although heteropolyacids are excellent homogeneous polyoxometalate catalysts, recovering these molecules at the end of a reaction is often tricky and can have an impact on their application. By inserting the polyoxometalate into the cavity of a metal-organic framework, scientists in Belgium have developed a way of releasing and re-trapping the catalyst at will.
The key to the system is to take advantage of the solvent-dependent solubility of the metal-organic framework. The team, led by Johan Martens from the University of Leuven, dissolved the caged catalyst, triggering the collapse of the metal-organic framework and releasing the heteropolyacid. They then used hexane to re-assemble the catalyst and metal-organic framework, producing a blue solid that could be removed easily by centrifugation.
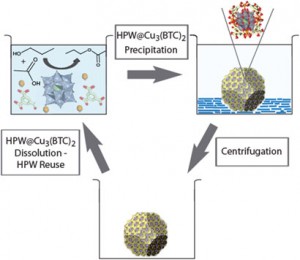
Recovery and reuse of a homogeneous catalyst through reversible encapsulation in a metal-organic framework
Read the full article in Chemistry World
Link to journal article
Recovery and reuse of heteropolyacid catalyst in liquid reaction medium through reversible encapsulation in Cu3(BTC)2 metal–organic framework
Nikki Janssens , Lik H. Wee , Sneha Bajpe , Eric Breynaert , Christine E. A. Kirschhock and Johan A. Martens
Chem. Sci., 2012, Advance Article, DOI: 10.1039/C2SC01102F