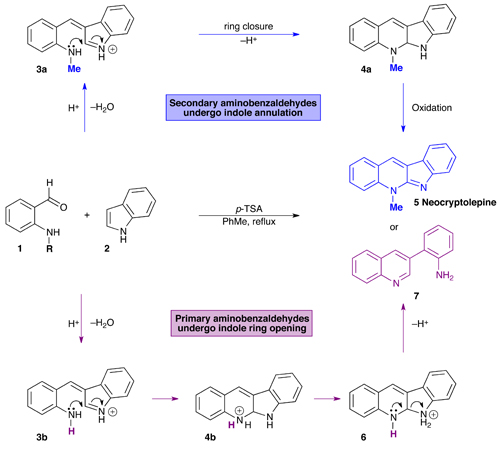
Researchers have shown that acid-catalysed condensations of aminobenzaldehydes with indole result in the formation of different products depending on whether the aminobenzaldehyde is primary or secondary.
Daniel Seidel’s group at Rutgers, The State University of New Jersey, found that N-methylbenzaldehyde reacts with indole to form neocryptolepine-related structures in a single step. Neocryptolepine (5) and its analogues are attractive targets for synthesis as they display promising antimalarial activity.
Conversely, primary benzaldehydes such as aminobenzaldehyde react under similar conditions to form quinolines (7). The mechanistic pathways leading to the formation of both indoles (5) and quinolines (7) are initially identical, with aminobenzaldehyde 1 condensing with indole 2 to form the corresponding azafulvenium ions (3), which undergo ring closure to yield tetracyclic products (4). At this point the mechanisms diverge; secondary systems (4a) undergo proton loss and oxidation to give neocryptolepine analogues (5), whilst primary systems (4b) undergo proton transfer to give products of type 6, which aromatise to form quinoline products (7) on ring opening.
The group made a range of both neocryptolepine analogues and quinoline systems in good to excellent yields, representing important scaffolds for medicinal chemistry.
“From the confirmation of the formation of neocryptolepine to the surprising results of the reactions of primary aminobenzaldehydes, this project was a fun experience with its intricacies and challenges” Aaron X. Sun, from the Seidel group
Seidel’s Edge article is free to download. Let me know what you think of this work by leaving your comments below.
Posted on behalf of Alice E. Williamson, Chemical Science web writer.