Photocatalysis of organic molecules in fatty acid membranes offers a plausible method for energy transfer and storage in prebiotic systems. We don’t know how early cells were formed – one suggestion is that the initial cell like structures were made through the self-assembly of fatty acids to form vesicles. However, this hypothesis still leaves many questions, including how these vesicles could harness energy for chemical reactions – an essential step for creating more complex systems.
In an Edge article published in Chemical Science, James Boncella and colleagues have reported that they developed a primitive energy transduction mechanism. Their research demonstrates that photocatalytic reactions involving polycyclic aromatic hydrocarbons (PAH) trapped in the vesicle membrane are capable of capturing and storing energy.
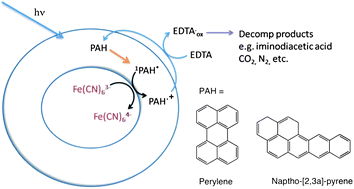
The vesicles are made from a hydrophobic membrane of fatty acids and polycyclic aromatic hydrocarbons surrounding an interior void containing metal anions. This membrane acts as barrier that prevents charged molecules from entering and leaving the vesicle. In a series of chemical reactions electrons are transferred across the membrane and trapped in charged molecules contained in the interior void. The cycle uses the PAH in the membrane as a photocatalyst to reduce Fe(CN)63- inside the vesicle. The PAH is then regenerated by oxidising EDTA molecules outside the cell which act as the electron source. Boncella explains that the cycle is a single electron process for harvesting energy. The next question is: “Can we use this energy to do useful chemistry?”
Unlike conventional methods to create vesicles the team used a complex mixture of short chain fatty acids and polycyclic aromatic hydrocarbons based on the composition of carbonaceous meteorites. The team hopes that these conditions mimic the environment found on earth thousands of years ago.
James Boncella reveals more about his work in a Chemical Science audio file, which accompanies his Edge article. Download it for free and let us know what you think of his research.