This month sees the following articles in Chemical Science that are in the top ten most accessed:-
Total synthesis of diazonamide A
Robert R. Knowles, Joseph Carpenter, Simon B. Blakey, Akio Kayano, Ian K. Mangion, Christopher J. Sinz and David W. C. MacMillan
Chem. Sci., 2011, 2, 308-311, DOI: 10.1039/C0SC00577K, Edge Article
Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide
David S. Surry and Stephen L. Buchwald
Chem. Sci., 2011, 2, 27-50, DOI: 10.1039/C0SC00331J, Perspective
Nitrogen-directed ketone hydroacylation: Enantioselective synthesis of benzoxazecinones
Hasan A. Khan, Kevin G. M. Kou and Vy M. Dong
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00469C, Edge Article
Ru-catalyzed activation of sp3 C–O bonds: O- to N-alkyl migratory rearrangement in pyridines and related heterocycles
Charles S. Yeung, Tom H. H. Hsieh and Vy M. Dong
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00498G, Edge Article
Ir-catalyzed highly selective addition of pyridyl C-H bonds to aldehydes promoted by triethylsilane
Bi-Jie Li and Zhang-Jie Shi
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00419G, Edge Article
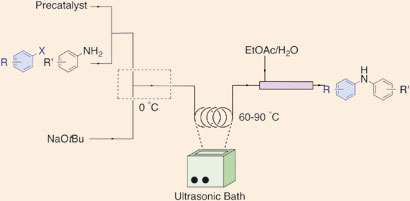
Palladium-catalyzed amination reactions in flow: overcoming the challenges of clogging via acoustic irradiation
Timothy Noël, John R. Naber, Ryan L. Hartman, Jonathan P. McMullen, Klavs F. Jensen and Stephen L. Buchwald
Chem. Sci., 2011, 2, 287-290, DOI: 10.1039/C0SC00524J, Edge Article
Palladium-catalyzed coupling of functionalized primary and secondary amines with aryl and heteroaryl halides: two ligands suffice in most cases
Debabrata Maiti, Brett P. Fors, Jaclyn L. Henderson, Yoshinori Nakamura and Stephen L. Buchwald
Chem. Sci., 2011, 2, 57-68, DOI: 10.1039/C0SC00330A, Edge Article
Sulfonated graphene as water-tolerant solid acid catalyst
Junyi Ji, Guanghui Zhang, Hongyu Chen, Shulan Wang, Guoliang Zhang, Fengbao Zhang and Xiaobin Fan
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00484G, Edge Article
Mechanised materials
Megan M. Boyle, Ronald A. Smaldone, Adam C. Whalley, Michael W. Ambrogio, Youssry Y. Botros and J. Fraser Stoddart
Chem. Sci., 2011, 2, 204-210, DOI: 10.1039/C0SC00453G, Minireview
Synthesis of boron doped polymeric carbon nitride solids and their use as metal-free catalysts for aliphatic C-H bond oxidation
Yong Wang, Haoran Li, Jia Yao, Xinchen Wang and Markus Antonietti
Chem. Sci., 2011, Advance Article, DOI: 10.1039/C0SC00475H, Edge Article
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Chemical Science? Then why not submit to us today or alternatively email us your suggestions.