US chemists have synthesised an entire family of marine alkaloids that show potent anticancer activity and potential for treating Alzheimer’s disease.
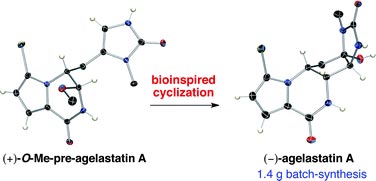
Mohammad Movassaghi and colleagues at Massachusetts Institute of Technology, Cambridge, were intrigued by the molecular architecture of agelastatin alkaloids, in particular the cyclopentane C-ring, which others had proposed is formed at an early stage in the alkaloid’s biosynthesis. Conversely, Movassaghi envisaged a biosynthetic sequence where the C-ring forms at an advanced stage of the synthesis and so he designed a total synthesis plan inspired by his biogenetic hypothesis.
This unique approach gave Movassaghi access to all known members of this alkaloid family. Although 10 different research groups had made agelastatin A before, Movassaghi’s route was the shortest, most efficient and largest scale synthesis to date, generating over 1.4 g of the highly potent antitumour agent.
To find out more about the new transformations developed, including an imidazolone-forming annulation reaction and a carbohydroxylative trapping of imidazolones, read the Chemical Science Edge Article.