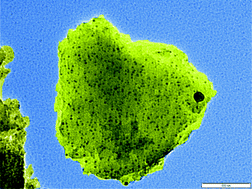
One goal of nanoparticle synthesis is to produce the lowest possible polydispersity – in other words, the best possible average size distribution. ‘Greener’ routes to achieve this without harmful solvents and capping agents often come from the natural world. In this case Graham Hutchings at Cardiff University, UK, plus collaborators in Bristol, UK, and Niigata, Japan, have used chitosan, a derivative of natural chitin found in crab and shrimp shells, to template the formation of supported gold-palladium nanoparticles.
These precious alloys are used as catalysts in the solventless aerobic oxidation of benzyl alcohol to benzaldehyde, an important class of reactions in the fine chemicals industry.
Hutchings and co-workers have previously found supported gold-palladium catalysts to be most active and stable for a range of other reactions including direct synthesis of hydrogen peroxide from H2 and O2, and for the oxidation of polyols such as glycerol. It is to be hoped that the new chitosan-templated synthesis will pave the way for new, greener routes to commercial scale production of fine chemical intermediates, for example allowing the replacement of traditional oxygen donors like chromate or permanganate.
To find out more, read about the work in RSC Advances for free:
Biotemplated synthesis of catalytic Au–Pd nanoparticles, Simon R. Hall, Andrew M. Collins, Natalie J. Wood, Wataru Ogasawara, Moataz Morad, Peter J. Miedziak, Meenakshisundaram Sankar, David W. Knight and Graham J. Hutchings, RSC Adv., 2012, 2, 2217–2220
Stay up-to-date with the latest content in RSC Advances by registering for our free table of contents alerts.
By Sara Coles