This month sees the following articles in Polymer Chemistry that are in the top ten most accessed for February:
Synthesis of Biodegradable Polymers from Renewable Resources
Mathieu J.-L. Tschan, Emilie Brulé, Pierre Haquette and Christophe M. Thomas
Polym. Chem., 2012, 3, 836-851
DOI: 10.1039/C2PY00452F
Diels-Alder “Click” Reactions: Recent Applications in Polymer and Material Science
Mehmet Atilla Tasdelen
Polym. Chem., 2011, 2, 2133-2145
DOI: 10.1039/C1PY00041A
Thiol-ene “click” reactions and recent applications in polymer and materials synthesis
Andrew B. Lowe
Polym. Chem., 2010, 1, 17-36
DOI: 10.1039/B9PY00216B
Terminal functional glycopolymers via a combination of catalytic chain transfer polymerisation (CCTP) followed by three consecutive click reactions
Qiang Zhang, Stacy Slavin, Mathew W. Jones, Alice J. Haddleton and David M. Haddleton
Polym. Chem., 2012, 3, 1016-1023
DOI: 10.1039/C2PY20013A
Hierarchical Self-Assembly Structures of POSS-Containing Polypeptide Block Copolymers Synthesized Using a Combination of ATRP, ROP and Click Chemistry
Yung-Chih Lin and Shiao-Wei Kuo
Polym. Chem., 2012, 3, 882-891
DOI: 10.1039/C2PY00574C
Conjugated Polymer Nanostructures for Organic Solar Cell Applications
Jiun-Tai Chen and Chain-Shu Hsu
Polym. Chem., 2011, 2, 2707-2722
DOI: 10.1039/C1PY00275A
Preparation of Jellyfish-Shaped Amphiphilic Block-Graft Copolymers Consisting a Poly(ε-caprolactone)-block-Poly(pentafluorostyrene) Ring and Poly(ethylene glycol) Lateral Brushes
Tao Cai, Wen Jing Yang, Koon-Gee Neoh and En-Tang Kang
Polym. Chem., 2012, 3, 1061-1068
DOI: 10.1039/C2PY00609J
Metal-Free Click Polymerization of Propiolates and Azides: Facile Synthesis of Functional Poly(aroxycarbonyltriazole)s
Hongkun Li, Jian Wang, Jing Zhi Sun, Rongrong Hu, Anjun Qin and Ben Zhong Tang
Polym. Chem., 2012, 3, 1075-1083
DOI: 10.1039/C2PY00586G
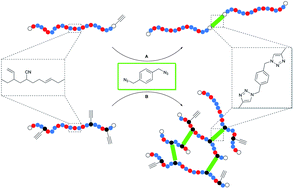
High molecular weight acrylonitrile-butadiene architectures via a combination of RAFT polymerization and orthogonal copper mediated azide-alkyne cycloaddition
Christoph J. Dürr, Sebastian G. J. Emmerling, Paul Lederhose, Andreas Kaiser, Sven Brandau, Michael Klimpel and Christopher Barner-Kowollik
Polym. Chem., 2012, 3, 1048-1060
DOI: 10.1039/C2PY00547F
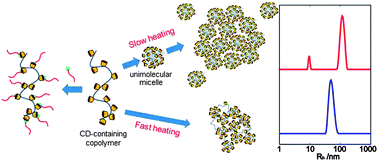
Polysaccharide-stabilized Core Cross-linked Polymer Micelle Analogues
Daniel J. Krasznai, Timothy F. L. McKenna, Michael F. Cunningham, Pascale Champagne and Niels M. B. Smeets
Polym. Chem., 2012, 3, 992-1001
DOI: 10.1039/C2PY00601D
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Polymer Chemistry? Then why not submit to us today!














 Henri Cramail received his engineering degree from the Ecole Nationale Supérieure de Chimie et de Physique de Bordeaux in 1987. He obtained his PhD from the LCPO, University of Bordeaux 1, in 1990 for studies in the field of ring-opening metathesis polymerization under the supervision of Profs Alain Soum and Michel Fontanille. After a post-doctoral stay with Prof. W.J. Feast at the University of Durham, U.K., he became an Assistant Professor of Polymer Chemistry at the University of Bordeaux 1 and, since 1999, he has been appointed Professor of Polymer Chemistry at the same University. In 2004, he was awarded the position of Junior Member of the ‘Institut Universitaire de France’. Since 2007, he is the Director of the Laboratoire de Chimie des Polymères Organiques (LCPO). His research interests concern (i) the coordination polymerization of olefins with a specific focus on single-site catalyst organic supports, (ii) step-growth polymerizations in dispersed media (organic phase, water, super critical CO2) to design core-shell particles with specific properties and, more intensively today, (iii) the development of new bio-based polymers from renewable resources (vegetable oils, terpenes) through green pathways (catalysis and processes).
Henri Cramail received his engineering degree from the Ecole Nationale Supérieure de Chimie et de Physique de Bordeaux in 1987. He obtained his PhD from the LCPO, University of Bordeaux 1, in 1990 for studies in the field of ring-opening metathesis polymerization under the supervision of Profs Alain Soum and Michel Fontanille. After a post-doctoral stay with Prof. W.J. Feast at the University of Durham, U.K., he became an Assistant Professor of Polymer Chemistry at the University of Bordeaux 1 and, since 1999, he has been appointed Professor of Polymer Chemistry at the same University. In 2004, he was awarded the position of Junior Member of the ‘Institut Universitaire de France’. Since 2007, he is the Director of the Laboratoire de Chimie des Polymères Organiques (LCPO). His research interests concern (i) the coordination polymerization of olefins with a specific focus on single-site catalyst organic supports, (ii) step-growth polymerizations in dispersed media (organic phase, water, super critical CO2) to design core-shell particles with specific properties and, more intensively today, (iii) the development of new bio-based polymers from renewable resources (vegetable oils, terpenes) through green pathways (catalysis and processes).