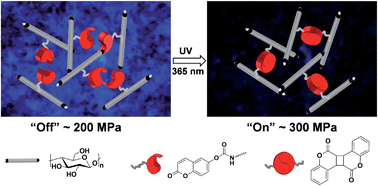
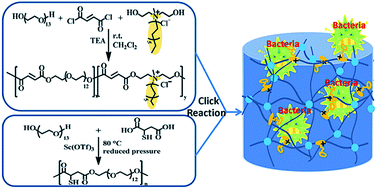
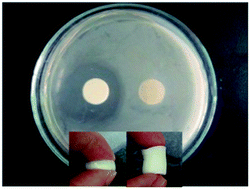
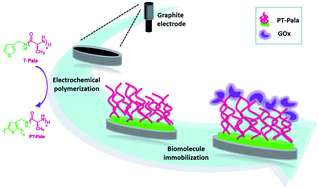
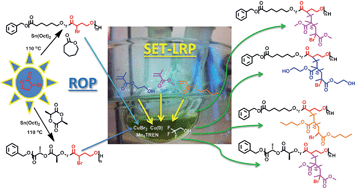
Exploiting the coumarin dimerization mechanism upon UV irradiation at 365 nm, Foster and co-workers developed mechanically adaptive nanocomposites in which light can be used to change the materials properties. CNCs derived from tunicates were functionalized with 7-coumaryl-(6-isocyanatohexyl) carbamate to afford coumarin-derivatized CNCs (Cou-CNCs). Light-responsive nanocomposites were prepared by reinforcing a rubbery ethylene oxide–epichlorohydrin copolymer (EO–EPI) matrix with Cou-CNCs. The as-prepared nanocomposites show a significantly increased tensile storage modulus (E′) in comparison to the neat EO–EPI. The optically induced reaction between Cou-CNCs also reduced the swelling of the EO–EPI/Cou-CNC nanocomposites upon exposure to water as well as the extent of water-induced softening.
Photoswitchable nanocomposites made from coumarin-functionalized cellulose nanocrystals by Mahesh V. Biyani, Christoph Weder and E. Johan Foster Polym. Chem. 2014, 5, 5501-5508.
Julien Nicolas is a web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.