 John (Jung Kwon) Oh is currently appointed as a Canada Research Chair (CRC) Tier II in Nanobioscience and an Assistant Professor in the Department of Chemistry and Biochemistry at Concordia University in Montreal, Canada. With BSc and MSc from Hanyang University in Korea, he earned his PhD degree from the University of Toronto in the area of polymer chemistry and materials science under the supervision of Prof. Mitchell A. Winnik. He then completed his postdoctoral research at Carnegie Mellon University with Prof. Kris Matyjaszewski, learning atom transfer radical polymerization (ATRP). He has been employed at Korea Chemical Company in Korea and Dow Chemical Company in Michigan USA over 10 years.
John (Jung Kwon) Oh is currently appointed as a Canada Research Chair (CRC) Tier II in Nanobioscience and an Assistant Professor in the Department of Chemistry and Biochemistry at Concordia University in Montreal, Canada. With BSc and MSc from Hanyang University in Korea, he earned his PhD degree from the University of Toronto in the area of polymer chemistry and materials science under the supervision of Prof. Mitchell A. Winnik. He then completed his postdoctoral research at Carnegie Mellon University with Prof. Kris Matyjaszewski, learning atom transfer radical polymerization (ATRP). He has been employed at Korea Chemical Company in Korea and Dow Chemical Company in Michigan USA over 10 years.
The research in his laboratory at Concordia enables the design and processing of macromolecular nanoscale materials for biological and biomedical applications. The nanomaterials of interest consist of polymeric, organic, and inorganic materials as well as hybrids having unique structural, electronic, magnetic, and optical properties. They are prepared by well-defined synthetic organic methods, controlled polymer chemistry as well as by templating with supramolecular assemblies. In particular, his interests are the integration of nanostructured biomaterials with biomedicine to develop advanced bionanomaterials that can interface biological processes as well as to understand their biological functions. The current focus of his research is on the development of a variety of novel biomaterials for drug delivery, cellular imaging, and tissue engineering, including superparamagnetic nanogels, rapid thermoresponsive hydrogels, and self-assembled degradable block copolymer micelles
Currently, his group has seven graduate students and postdoctoral fellows. The students and postdoc in his laboratory who participate in the cutting-edge research program have the opportunity to gain a broad range of skills and knowledge in organic polymer chemistry, materials chemistry and science, and the domain of biology and biomedical engineering.
What was your inspiration in becoming a chemist?
My impression about synthetic chemistry is that a synthetic chemist is similar to a professional cook who can select appropriate and various ingredients to create new tastes. I have trained as polymer chemist and materials scientist in both industrial and academic settings. My strong desire is to integrate my synthetic skill sets with biology and biomedicine, developing new materials for biomedical research.
What was the motivation behind the research in your recent Polymer Chemistry paper? (DOI:10.1039/c2py20154b)
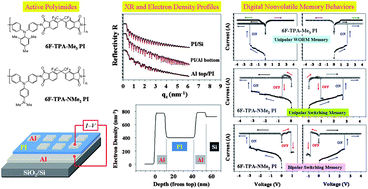
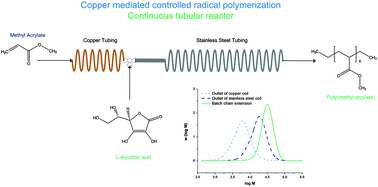
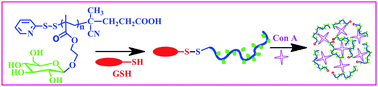
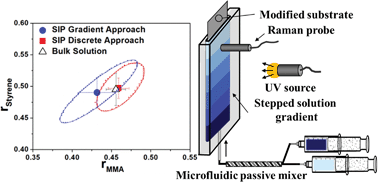
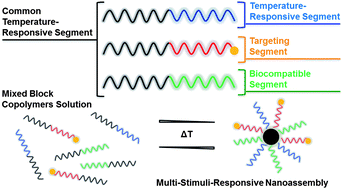
Toward the effective cancer-targeting drug delivery applications of amphiphilic block copolymers as multifunctional nanocarriers, the control of the release of encapsulated anticancer therapeutics is critical. My research group has focused on the new design of stimuli-responsive degradable self-assembled micelles, with different numbers and types of degradable linkages positioned at various locations. These micelles having topological variations should allow the determination of the structure-property relationship between morphological variance and stimuli-responsive degradation. Ultimately, the advanced knowledge can be leveraged into optimizing degradable micelles offering tunable release of encapsulated anticancer therapeutics inside cancer cells.
In which upcoming conferences may our readers meet you?
I regularly attend the ACS meeting in USA and will attend the 95th Canadian Chemistry Conference and Exhibition in Calgary in Canada.
How do you spend your spare times?
I walk with my wife and love to play tennis.
Which profession would you choose if you were not a scientist?
I loved and played baseball in my childhood. Because I was born in countryside in the city of Daejeon, Korea, I did not have an great opportunity to be a baseball player in my life. If I am given to a new life, I would be a professional baseball player.