Professor Jürgen Liebscher graduated from Technical University Dresden, Germany where he also obtained his PhD and habilitation (1977). From 1979 till 1982 he held the position of Associate Professor at Addis Ababa University, Ethiopia and joined the Department of Chemistry, Humboldt-University Berlin later on, where he is professor of organic chemistry. Since 2010 he has been Senior Researcher I and project leader at the National Institute for Research and Development of Isotopic and Molecular Technologies (IN CDTIM) in Cluj-Napoca, Romania and Assoc. Professor at Babes Bolyai University Cluj-Napoca, Faculty of Chemistry and Chemical Engineering. His research interests are widespread ranging from organic synthesis, bioorganic chemistry (nucleic acid-lipid conjugates, amino acids), catalysis, peroxide chemistry, heterocycles to pharmaceutically active compounds. For 10 years he has mainly been involved in the field of materials, i. e. magnetic core-shell nanoparticles, where he entered polymer chemistry, in particular polymers with functional groups for various applications (catalysis, biological recognition, separation, drug transport and delivery).
Professor Jürgen Liebscher graduated from Technical University Dresden, Germany where he also obtained his PhD and habilitation (1977). From 1979 till 1982 he held the position of Associate Professor at Addis Ababa University, Ethiopia and joined the Department of Chemistry, Humboldt-University Berlin later on, where he is professor of organic chemistry. Since 2010 he has been Senior Researcher I and project leader at the National Institute for Research and Development of Isotopic and Molecular Technologies (IN CDTIM) in Cluj-Napoca, Romania and Assoc. Professor at Babes Bolyai University Cluj-Napoca, Faculty of Chemistry and Chemical Engineering. His research interests are widespread ranging from organic synthesis, bioorganic chemistry (nucleic acid-lipid conjugates, amino acids), catalysis, peroxide chemistry, heterocycles to pharmaceutically active compounds. For 10 years he has mainly been involved in the field of materials, i. e. magnetic core-shell nanoparticles, where he entered polymer chemistry, in particular polymers with functional groups for various applications (catalysis, biological recognition, separation, drug transport and delivery).
Links to his research groups are: http://www.itim-cj.ro/~liebscher/ (and before at Humboldt-University Berlin: http://fakultaeten.hu-berlin.de/mnf1/mitarbeiter/4125.)
What was your inspiration in becoming a chemist?
I had an excellent chemistry teacher (Erhard Matthes) at high school in Freital, Germany, who made me highly interested in this field. I still remember some highlights (tasting synthetic urea, distillation of alcoholic drinks, where half of the class was tipsy before we even started the experiment, explosion of phosphorous-perchlorate mixture, preparation and investigations of simple polymers). He is now 91 years old and a dear friend of mine with whom I still enjoy exciting discussions during our visits. I like chemistry because of its interaction of practical experiments with theoretical backgrounds. It is exciting to have an idea, to go to the laboratory and get it verified experimentally. Because most of the ideas do not work, it makes the final success even more attractive. Chemistry is also interesting to me because of its potential of practical application, to find something that is useful in our society. In this respect, interdisciplinary research is essential and challenging. I learned a lot and shaped my scientific profile much by such collaborations as I learned from lecturing students in advanced organic chemistry courses.
What was the motivation to write your Polymer Chemistry article?
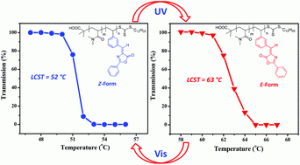
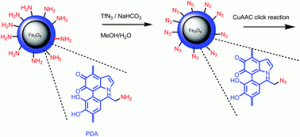
Our group got new insight into the structure of polydopamine, a material which after its invention by Messersmith et al. in 2007 is in the focus of contemporary research. We succeeded to provide experimental proof by spectroscopic methods that this material contains primary amino groups, which hitherto were not exploited for interesting synthetic modifications. Recently we found that polydopamine even acts as an organocatalyst, i.e. it is not an innocent polymer. As we learned from the responce of reviewers to our publication manuscripts not everybody shared our opinion. The successful diazo transfer reaction to dopamine reported in our Polymer Chemistry article is further unambiguous experimental proof for the existence of primary amino groups in polydopamine. In addition, it opens a way to link a variety of interesting functions (catalytic, biological, complex forming) to polydopamine via click chemistry (CuAAC). It further allows Janus like systems with at least two different functions connected to polydopamine.
Why did you choose Polymer Chemistry to publish your work?
Polymer Chemistry is a journal of high quality. It attracts a wide readership by covering chemistry and the polymer field. We find that scientists who are interested in our results will be reached by this journal. The time between submitting the manuscript and receiving the decision about its acceptance is usually very short thanks to the well organized editorial and production team.
In which upcoming conferences may our readers meet you?
4th International Conference on Multifunctional, Hybrid and Nanomaterials, March 2015, Sitges (near Barcelona), Spain.
How do you spend your spare time?
I dedicate most of my free time to my 1 year old son. When he gets a bit older I will return to my hobbies such as enjoying nature, hiking, biking and classical concerts.
Which profession would you choose if you were not a chemist?
It is hard for me to imagine another profession which would give me so much satisfaction. Maybe, I would choose biochemistry, biology or eventually medicine.

Read Jürgen’s latest paper:
Diazo transfer at polydopamine – a new way to functionalization
Polym. Chem., 2014, Advance Article, DOI: 10.1039/C4PY00670D
Cyrille Boyer is a guest web-writer for Polymer Chemistry. He is currently an associate professor and an ARC-Future Fellow in the School of Chemical Engineering, University of New South Wales (Australia) and deputy director of the Australian Centre for NanoMedicine.
Comments Off on Author of the Month: Jürgen Liebscher















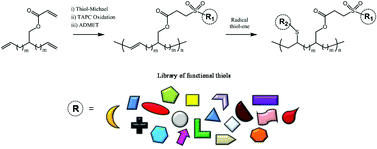
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.
C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.