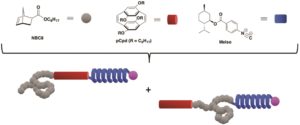
Natural proteins are comprised of distinct secondary structure elements such as sheets, helices and coils. It is the combination of these diverse topologies that allow proteins to fulfil their functions. Owing to the properties of these structures, synthetic analogues of these materials are also of great interest to the polymer chemistry community. However, a covalent system where sheet, helix, and coil blocks are combined in a linear system has not been yet realized. Towards this direction, Weck, Elacqua and co-workers developed a new methodology to covalently link three distinct structures together with high fidelity without compromising the control over sequence. This was achieved by combining sequential ring-opening metathesis polymerization (ROMP) of sheet- and coil-forming monomers with palladium-mediated isocyanide polymerization of covalent coil-sheet-helix (ABC) and sheetcoil helix (BAC) domains. After polymerizing the initial sheet of coil-forming monomer through ROMP, the second monomer is introduced and subsequently polymerized yielding to a diblock comprised of sheet and coil structures. ROMP is then terminated by a special transfer agent that contains an isocyanide polymerization initiator. The telechelic diblock copolymers containing both coil-sheet and sheet-coil blocks, can serve as macroinitiators for the polymerization of a P-helix forming monomer. As such, this combination of sequential copolymerization and macroinitiation enables three different polymer chains to be linked covalently. Importantly, throughout the triblock copolymer synthesis, all individual blocks retained their secondary structures as evidenced by circular dicroism and fluorescence spectroscopies. The authors are confident that this work can be extended to the formation of a diverse array of tri- and multiblock copolymers enabling a range of new applications.
Tips/comments directly from the authors:
1. When synthesizing a topologically-diverse block copolymer, oftentimes it is necessary to use different polymerization techniques. If so, prudent selection and design of polymer backbone is key. First, select the class(es) of monomers you intend to employ. This will inform the type of polymerization method required, and subsequently, the initiator to be designed.
2. Ring-opening metathesis polymerization (ROMP) is a widely-used controllable polymerization method that allows for one to not only control molecular weight, but also is amenable to an iterative or tandem ROMP, which is desirable for sequence-controlled block copolymers
3. Performing 31P NMR spectroscopy after each step of block copolymer synthesis, especially before the final step to create the helical block, is crucial. It ensures that only one palladium species is present throughout.
4. Isocyanide polymerization mediated by palladium(II) is a robust technique; there is high functional group tolerance when synthesizing the initiator, which allows for the engineering of multipurpose catalysts like the one featured in this manuscript.
5. Topologically-diverse polymer backbones, such as sheets, helices, and coils, garner much interest from a biomimetic standpoint in the synthetic community. Judicious choice of polymer backbones, as well as block lengths, can inform characterization techniques, such as circular dichroism, fluorescence, and X-ray scattering to gain insights into topology.
6. We are available for any questions and to troubleshoot any issues you may have – please contact mw125@nyu.edu or eze31@psu.edu.
Synthesis of sheet-coil-helix and coil-sheet-helix triblock copolymers by combining ROMP with palladium-mediated isocyanide polymerization, Polym. Chem., 2018, 9, 5655-5659, DOI: 10.1039/C8PY01361F
About the webwriter
 Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently an Assistant Professor at ETH Materials Department.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently an Assistant Professor at ETH Materials Department.