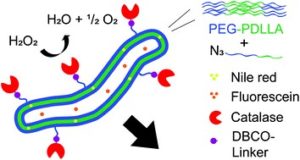
The potential of nanomachines to mimic biological systems that can continuously move in and between cells to perform a function has attracted significant attention over the past decades. Although the autonomous movement, speed and functionality of various artificial nanomotors has improved over the years, it is interesting to note that the vast majority of them are based on catalytically active metals and harsh metal surfaces while requiring toxic fuel for propulsion. Wilson and co-workers are the first to report a biodegradable nanomotor which can autonomously move in the presence of fuel while carrying a load. To achieve this, Wilson’s group created poly(ethylene glycol)-b-poly(D,L-lactide) (PEG-PDLLA) polymerosomes with 5% wt% functional azide handles by employing ring opening polymerization. The spherical polymerosomes were then transformed to nanotubes by inducing osmotic pressure. The azide handles presented in periphery of the nanotubes were converted into COOH groups using strain-promoted alkyne-azide cycloaddition. Finally, catalase was coupled to the nanotubes surface via EDC coupling. Importantly, the catalytic conversion of H2O2 by the enzyme provided adequate propulsion to move the nanotubes forward. In addition, both hydrophobic and hydrophilic drugs could be simultaneously loaded in the tubes. Given the advantageous characteristics of tubular-shaped polymersomes, such as high-aspect-ratio and higher loading capacity, such materials can be potentially used as excellent nanocarriers for drug delivery.
Tips/comments directly from the authors:
- For the synthesis of PEG-PDLLA, keep the ratio of catalyst to initiator bellow 0.5 equivalents to obtain polymers with low PDI. Furthermore, the reaction is oxygen and water sensitive and should thus be carried out in inert atmosphere.
- The formation of polymersomes requires stable conditions, as small changes can affect the morphology and the polydispersity of the vesicles. Pre-cooled water is used for the shape transformation by dialysis while the dialysis is carried out in in the fridge at 4°C.
- Centrifugation of the nanotubes for functionalization reaction should not exceed 5000 rpm and should not be longer than 10 min to prevent aggregation and clogging of the spin filter and breakage of the tubes.
- It is recommended to use low concentration of nanomotors (< 109 particles/ml) when measuring their movement with the Nanosight LM10, as high oxygen production can lead to drift of the sample (dilute such that single motors are visualized).
Enzyme-driven biodegradable nanomotor based on tubular-shaped polymeric vesicles, Polym. Chem., 2018, 9, 3190-3194, DOI: 10.1039/C8PY00559A
This paper is free to read and download until 6 August!
About the webwriter
 Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit http://hawkergroup.mrl.ucsb.edu/members/athina-anastasaki for more information.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit http://hawkergroup.mrl.ucsb.edu/members/athina-anastasaki for more information.