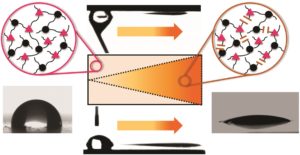
Controlled radical polymerization strategies are often exploited to tailor the properties of functional polymer networks. With the recent developments in external stimuli to regulate polymerization, the use of light has received significant attention as it enables the synthesis of materials with precise spatial, temporal, and sequence control. In order to design structurally tailored and engineered macromolecular (STEM) networks, Matyjaszewski and co-workers proposed a new, metal-free approach to prepare well-defined networks. To achieve this, the authors selectively activated the fragmentation of trithiocarbonate reversible addition-fragmentation chain-transfer (RAFT) agents by visible light RAFT iniferter photolysis coupled with RAFT addition-fragmentation process. Through this two-step synthesis, different materials could be polymerized yielding compositionally and mechanically differentiated networks. Upon carefully selecting the crosslinker as well as the RAFT inimer, three different types of primary polymethacrylate networks could be generated under green light. The obtained networks were further enriched by the addition of methyl acrylate and dimethylacrylamide under blue light, resulting in soft and stiff gels respectively. Importantly, dynamic mechanical analysis was utilized to characterize the mechanical properties of both the starting and the final materials and to determine their glass transition temperatures. Such STEM networks significantly expand the toolbox of polymer and material science.
Tips/comments directly from the authors:
- Structurally tailored and engineered macromolecular (STEM) networks are versatile materials containing latent functional groups accessible for post-synthesis modifications to afford new chemical and material properties.
- The network synthesis and modifications were controlled using dual wavelengths (green and blue). The primary network was synthesized under green light irradiation, and the subsequent modifications were performed under blue light.
- Initial network synthesis involves incorporation of two RAFT photoiniferters with similar Z groups (thioalkyl) but different R groups (either a tertiary or secondary carbon radical) to enable activation of one RAFT agent over the other under green light. This is followed by activation of both RAFT agents for secondary modification under blue light.
- The n to π* electronic transition at 520 nm affords photolysis of trithiocarbonate with 4-cyanopentanoic acid R-leaving group under green light leading to generation of tertiary carbon radicals promoting polymerization of methacrylates. The second trithiocarbonate RAFT agent with propionic acid R-leaving group is also incorporated into this network during this process as a RAFT methacrylate monomer or dimethacrylate crosslinker.
- Selective activation under green light is made possible as the addition of 4-cyanopentanoicacid radical to trithiocarbonate RAFT agent with propionic acid R-leaving group does not lead to fragmentation as radical stabilization energies of tertiary radicals are higher than secondary radicals.
- Therefore, the methacrylate/dimethacrylate RAFT agent with propionic acid R-leaving group remains inert under green light and can only be activated under blue light (465 nm) where the n to π* electronic transition lies.
- Both RAFT agents (secondary and tertiary leaving groups) are then activated in a second step which involves soaking in a second monomer (acrylate or acrylamides) into the network followed by polymerization under blue light.
- Depending on the functionality of the second monomer, the post-modified network can be either softer or stiffer with different responses to polarity (hydrophilicity/hydrophobicity).
Read the full paper now for FREE until 12th July!
Transformation of gels via catalyst-free selective RAFT photoactivation, Polym. Chem., 2019, 10, 2477-2483, DOI: 10.1039/C9PY00213H
About the webwriter
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.


![c9py00213h-ga[1]](https://blogs.rsc.org/py/files/2019/06/c9py00213h-ga1-300x124.jpg)