Now that 2022 has come to an end, join us as we look back at some of the highlights of last year and forward to our upcoming activities in 2023!
Polymer Chemistry Top Picks of 2022
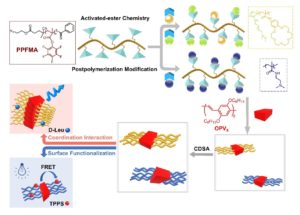
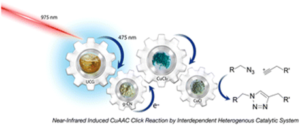
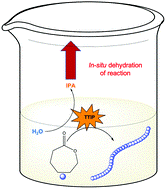
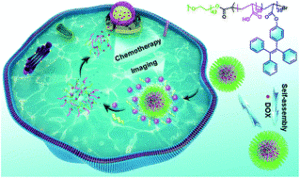
We have selected some of the most cited, most downloaded and most shared articles published in Polymer Chemistry from the last year for our Most Popular 2022 collection.
All articles in this collection are FREE to read until 28 February 2023.
Congratulations to all featured authors!
Editorial Board
We would like to thank Professor Wei You for his support of Polymer Chemistry as he stepped down from his role on the Editorial Board at the end of 2022.

| Polymer Chemistry Lectureship
The Polymer Chemistry Lectureship 2022 was awarded to Professor Dominik Konkolewicz (Miami University, USA). This annual award was established in 2015 to honour an early-stage career scientist who has made a significant contribution to the polymer field. The Konkolewicz group explores a range of topics in polymer chemistry, including radical polymerisation mechanisms, dynamically bonded polymer materials, light driven reactions, bioconjugates and polymer based self-assembly. Find out more about Dominik and his research on our Lectureship winner blog post. You can check out articles from Dominik and our previous winners in the Lectureship winners collection. |
 |
Did you know that nominations for the Polymer Chemistry Lectureship 2023 are now open? If you know an outstanding early career researcher in polymers, nominate them before the 28 February 2023.
Full details about eligibility and the nominations process can be found here

Polymer Chemistry Emerging Investigators
Polymer Chemistry is proud to spotlight our ongoing Emerging Investigators Series. Our Emerging Investigators are at the early stages of their independent careers and invited for this collection in recognition of their potential to influence future directions in the field. Congratulations to all the featured researchers on their important work so far!
Read the collection
Meet the Scientists
Themed collections
Polymer Chemistry is delighted to have featured some of your best work in our themed collections in 2022.
Check out some of these ongoing collections:
Browse all past collections on our platform and see our upcoming collections on our calls for submissions page. We will be announcing more collections during the year, so keep a look out!
HOT articles
Remember to check out our ongoing Polymer Chemistry HOT articles collection featuring articles highlighted by our Editors and referees. All articles in the collection are FREE to read until 28 February 2023.

| Paper of the Month blogs
Our Web Writer and Advisory Board member Dr Kelly Velonia publishes a blog highlighting an interesting publication of her choice each month. She summarises the work and interviews the authors for tips and comments about their work.
Check out the ‘Paper of the Month’ blogs for 2022 here |
 |
Open Access
The Royal Society of Chemistry has announced that all 31 fully-owned hybrid journals, including Polymer Chemistry, have been approved as “Transformative Journals” with cOAlition S, an international consortium of research funding and performing organisations. Find out more about our strive towards 100% Open Access here.
#RSCPoster: Save the date
| #RSCPoster is a global Twitter Poster Conference, held entirely online over the course of 24 hours. The event brings together the global chemistry community to network with colleagues across the world and at every career stage, share their research and engage in scientific debate.
The 2023 #RSCPoster Twitter Conference will be held from 12:00 (UTC) 28 February 2023 to 12:00 (UTC) 1 March 2023. |
 |
How you can help…
We would like to take this opportunity to thank all of you in addition to our authors, reviewers and readers for their support throughout 2022. Here are some of the ways in which you can continue to make a positive contribution to Polymer Chemistry:
Submit to one of our open themed collections and encourage your colleagues to submit.
If you are organising a conference or virtual event, please do let us know if you would like to arrange mutual promotion between the conference and Polymer Chemistry. We can offer poster prizes, social media and blog promotion, and adverts in the journal and on the journal web page.
Read our recent articles and follow the latest news on the Polymer Chemistry blog and on our Facebook and Twitter pages.
Send your best research to Polymer Chemistry.
Sign up to be a reviewer for Polymer Chemistry.
Thank you for your continued interest in and support of Polymer Chemistry. We look forward to seeing what 2023 brings!