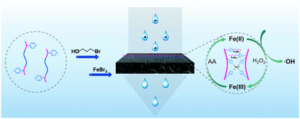
Guan et al. develop iron-containing poly(ionic liquid) (Fe-PIL) membranes with anti-fouling properties enhanced by a heterogeneous Fenton reaction.
The life span of membranes used in various separation technologies is often limited by fouling causing decreased performance and large economic costs. Recently, poly(ionic liquid)s (PILs) have been employed to prepare membranes with a large range of applications due to their unique material properties including excellent stability, processability and flexibility. Although PIL membranes are less prone to fouling and easier to clean due to their charged nature, the problem of irreversible pollutant deposition can limit their efficiency.
To address this, Zhang and collaborators developed iron-containing PIL (Fe-PIL) membranes and used them as catalysts for heterogenous Fenton reaction. Poly(4-vinylpyridine)-b-polysulfone-b-poly(4-vinylpyridine) (PSF-b-P4VP) blend membranes were synthesized via Cu(0)-RDRP. The pore size and hydrophilicity of the membranes fabricated via NIPS, were found to depend on the block ratio of the polymer. A quaternization reaction followed by coordination with Fe(II) bromide was employed to generate the Fe-PILs on the surface of the polysulfone blends. The membranes were shown to possess low surface roughness, increased hydrophilicity, anti-fouling properties and scalability. The dispersibility of the catalyst and the catalytic efficiency in heterogeneous Fenton reactions were shown to be excellent in a broad pH range from acidic to neutral and basic conditions. More importantly, the Fe-PIL membranes exhibited superior synergistic performance with filtration in the dynamic heterogeneous Fenton reaction and excellent reusability as they could be maintained well after five cycles.
In summary this study combines PIL membrane technology with dynamic heterogeneous catalysis (Fenton reaction) to create reusable PILs that address the issue of membrane fouling.
Iron-containing poly(ionic liquid) membranes: a heterogeneous Fenton reaction and enhanced anti-fouling ability, Polym. Chem., 2022,13, 130-138
Link to the paper: https://pubs.rsc.org/en/content/articlelanding/2022/py/d1py01345a
 Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.