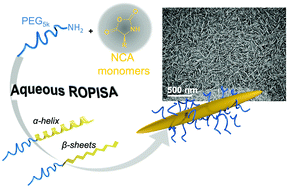
Bonduelle et al. prove that ROPISA can be used to concomitantly yield well-defined amphiphilic copolypeptide chains and self-assembled nanostructures in a rapid, facile, and straightforward manner.
Synthetic polypeptides are among the most versatile building blocks to guide the formation of self-assembled nanomaterials through biomimetic structuring since they merge advantageous features of both synthetic polymers and proteins. Recent advances in ring-opening polymerization methodologies offer the unique ability to precisely design polypeptides fitting a particular function. In this context, application of polymerization-induced self-assembly (PISA) – i.e. in situ growth of a living amphiphilic polymer chain during its self-assembly into nanostructures- offers unprecedented possibilities for the synthesis of functional peptide-based nanomaterials.
To address this possibility, Bonduelle, Lecommandoux and collaborators comprehensively studied the recently reported aqueous ROPISA process of N-carboxyanhydrides in the presence of α-amino-poly(ethylene oxide) initiators. A library of polypeptides was prepared from two NCA monomers derived from benzyl-L-glutamate (BLG-NCA) and L-Leucine (Leu-NCA) and a hydrophilic PEG5kDa-NH2 macroinitiator by varying the degree of polymerization. This combined one-pot synthesis and self-assembly was found to produce well-defined amphiphilic copolypeptide chains with narrow molar mass dispersity (Đ) values (between 1.12 and 1.17), controllable number-averaged molar mass Mn and good reaction yields. Importantly, in contrast to previous studies where nanomaterial morphology was essentially defined by the hydrophobic to hydrophilic ratio, the anisotropic rod-like nanostructure morphologies were dictated by the chemical nature of the NCA monomer and the secondary structure of the resulting polypeptides. In addition, ROPISA was found to provide control over the diameter of the produced rod-like self-assembled nanostructures. The β-sheet forming PLeu polypeptides were found to strongly favor the formation of long rods with high aspect ratio, as compared to α-helical PBLG polypeptides.
In summary, ROPISA provides a rapid, facile, and straightforward methodology for the synthesis of rigid polypeptide-based nanomaterials at high solid content with tunable anisotropy. These new results strengthen the potential of using ROPISA to obtain polypeptides with N-carboxyanhydrides and open new avenues towards the design of functional nanomaterials.
Tips/comments directly from the authors:
- The polymerization of NCAs via ROPISA can easily be contaminated by homopolymers that originate from the hydrolysis of a monomer to an amino acid that then initiates the polymerization process. Salt concentration and temperature are important parameters to control this secondary reaction.
- The agitation of the reaction medium is a key factor in the ROPISA process: insufficient stirring makes the synthesis too inhomogeneous and does not provide good results. At high solid contents (above 10%) and in some experiments, hydrogels are obtained, a phenomenon we are currently studying.
- Other molar mass of poly(ethylene glycol) can be used: we have performed ROPISA with PEG2k or PEG10k with the two monomers described. We obtain in most cases anisotropic nano-objects.
Citation to the paper: Aqueous ROPISA of α-amino acid N-carboxyanhydrides: polypeptide block secondary structure controls nanoparticle shape anisotropy, Polym. Chem., 2021,12, 6242-6251
Link to the paper: https://pubs.rsc.org/en/content/articlelanding/2021/py/d1py00995h
 Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers.