The emergence of the Click Chemistry concept has led to the identification of a set of highly efficient, reliable and selective reactions that readily meet the demands and desires for the synthesis of new materials. Specifically, the copper catalyzed azide–alkyne cycloaddition (CuAAC) reaction has been prominently established as the ideal click reaction due to its simplicity, orthogonality and regioselectivity relative to other organic reactions. Unlike conventional CuAAC approaches using Cu(I) salts, reducing agents, or copper turnings, a photo-mediated technique for generating Cu(I) in situ affords comprehensive spatial and temporal control of the CuAAC reaction, wherein the generation of Cu(I) is limited to selectively irradiated regions in both time and space. In addition to the spatiotemporal control, the photo-induced CuAAC reaction offers a facile means to control the reaction rate simply by changing the light intensity or photoinitiator concentration.
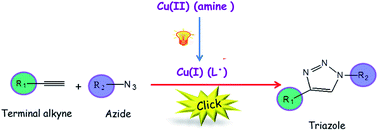
In this paper, Bowman and co-workers demonstrated both spatial and temporal control over the CuAAC by the photo-induced generation of the active form of the catalyst Cu(I) to initiate the CuAAC reaction. Tertiary aliphatic amine ligands were used as an electron transfer species to reduce Cu(II) upon irradiation while also functioning as an accelerating agent and as protecting ligands for the Cu(I). Moreover, this catalyst system was demonstrated to be effective, adaptable to organic and aqueous media, and highly selective to the CuAAC reaction.
Evaluation and development of novel photoinitiator complexes for photoinitiating the copper-catalyzed azide–alkyne cycloaddition reaction by Abeer A. Alzahrani, Annette H. Erbse and Christopher N. Bowman Polym. Chem. 2014, 5, 1874-1882.
Julien Nicolas is a web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.











