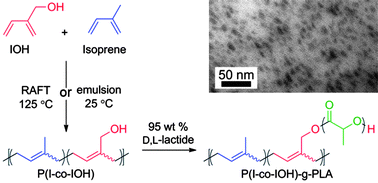
Polyisoprene (PI) is an interesting backbone material for graft copolymer synthesis. Isoprene can be renewably sourced and polymerized through a variety of methods. Hydroxyl functionalized PI is interesting as it allows for the synthesis of polylactide graft copolymers with a rubbery backbone, which can be quite tough materials as compared to the brittle poly(lactic acid) (PLA) homopolymer. In an effort to develop a hydroxyl functionalized PI macroinitiator, the authors investigated RAFT copolymerizations of isoprene with 2-methylenebut-3-en-1-ol (IOH) and with commercially available, hydroxyl containing monomers 2-hydroxyethyl acrylate (HEA) and 2-hydroxyethyl methacrylate (HEMA). The monomer IOH, structurally similar to isoprene, exhibited favorable kinetics towards copolymerization and copolymerized with isoprene in a rather random manner, producing P(I-co-IOH) macroinitiators with hydroxyl content close to that which was fed into the system. Additionally, IOH was copolymerized with isoprene in an emulsion process at room temperature. The hydroxyl containing isoprene copolymers were also able to undergo subsequent reactions efficiently to produce PLA graft copolymers.
Copolymerization of isoprene and hydroxyl containing monomers by controlled radical and emulsion methods by William M. Gramlich , Grayce Theryo and Marc A. Hillmyer Polym. Chem., 2012, 3, 1510-1516.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.