Polyoxometalates (POMs) are a diverse class of inorganic materials that are of great interest due to their exciting range of redox, conducting, magnetic and catalytic properties. Recent collaborative work from Professor Garry Hanan in Montreal and Professor Bernold Hasenknopf in Paris reports the inclusion of a Lindqvist-type hexavanadate POM as a component of a self-assembling supramolecular framework.
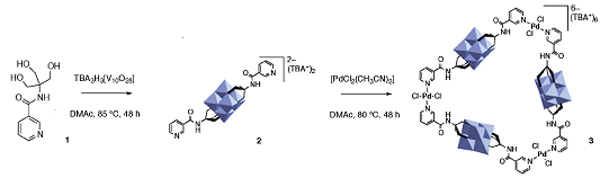
They designed ligand 1 utilising a triol motif to complex the POM in combination with pyridyl groups to serve as binding sites for a transition metal. The reaction of 1 with a suitable decavanadate yielded complex 2, a hexavanadate POM complex that is itself a structurally rigid and spatially well-defined bi-pyridyl ligand. The 60° angle between the coordination vectors of the pyridyl groups led the authors to predict that the coordination of a trans-PdCl2 unit by 2 would result in a supramolecular triangle.
Initially the reaction of 2 with [PdCl2(CH3CN)2] in DMAc yielded a complex mixture of products; however, heating to 80 °C for 48 hours led to just a single assembly. This was shown by a number of analytical techniques to be the predicted triangular assembly 3.
This work elegantly uses a classical motif for self-assembly to create a multi-component supramolecular architecture. It is a great step towards the goal of creating functional supramolecular arrays, integrating the desirable properties of POMs into a new framework and bridging the gap between solid state oxides and coordination chemistry.
To read more about Hanan and Hasenknopf’s work, download their ChemComm article.
Posted on behalf of Cally Haynes, ChemComm web writer.














 ….and did you know?….
….and did you know?….
 Xenon is naturally present in very small amounts in the atmosphere but radioactive forms are released following nuclear detonations, reprocessing and explosions, such as the recent catastrophe at Fukushima Daiichi Nuclear Power Plant in Japan. Xenon is also used in a variety of other applications, from lighting to medical imaging, so capturing and separating it (from its sister noble gas krypton) is important for both commercial uses and atmospheric monitoring.
Xenon is naturally present in very small amounts in the atmosphere but radioactive forms are released following nuclear detonations, reprocessing and explosions, such as the recent catastrophe at Fukushima Daiichi Nuclear Power Plant in Japan. Xenon is also used in a variety of other applications, from lighting to medical imaging, so capturing and separating it (from its sister noble gas krypton) is important for both commercial uses and atmospheric monitoring.