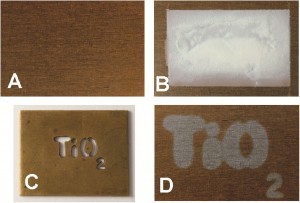
A simple and cheap way to clean tarnished metals using a UV activated photocatalyst ink has been developed by UK scientists. Removing steel corrosion is a major concern in many industries. Most metals are thermodynamically unstable in air and aqueous solution but owe their durability to metal oxides that form on the surface. However, when the metal oxides thicken, it leads to contamination with undesirable corrosion products that need to be removed. Aggressive chemicals such as strong acids and chelating agents are usually required to remove them.
The photocatalyst ink was applied to the tarnished metal and then UV light was shone onto it. This produced conduction band electrons and valence band holes. An electron donor species in the ink reacted with the holes, leaving the photogenerated electrons to react with absorbed metal ions. The metal oxide layer could then be removed with water.
Reference:
Photocatalyst film and ink for cleaning tarnished metals
A Mills and D Hazafy, Chem. Commun., 2011
DOI: 10.1039/c1cc15774d













 Chinese scientists have made a simple and sensitive sensor for detecting proteins, which could lead to improved disease detection.
Chinese scientists have made a simple and sensitive sensor for detecting proteins, which could lead to improved disease detection.