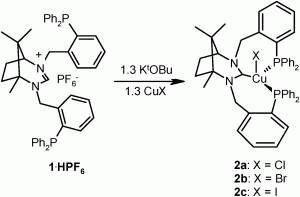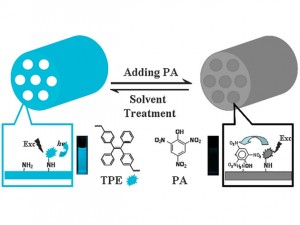
A mesoporous material functionalised with aggregation-induced emission (AIE) luminogens serves as an efficient and recyclable fluorescent sensor to detect picric acid (PA) in water
A reusable fluorescent sensor that detects explosives in groundwater or seawater could aid antiterrorist activities and environmental protection, say scientists in China.
Many techniques have been used to detect explosives, including gas chromatography, Raman spectroscopy and fluorescence spectroscopy. The latter is considered the most useful because of its simplicity, high sensitivity and low cost. With this technique, fluorescent dyes are incorporated into a solid matrix on which they interact with the explosive molecules, causing them to fluoresce.
However, the dyes often aggregate within the matrix, leading to a substantial decrease in fluorescence, known as quenching. This loss of efficiency and sensitivity motivated the discovery of molecules whose fluorescence is turned on by aggregation rather than suppressed. Materials based on such aggregation-induced emission (AIE) luminogens (luminescent compounds) have shown promise in explosive detection in organic solvents. Dispersion in aqueous solution has, until recently, proven difficult.
Read the full article in Chemistry World
Link to journal article
Supersensitive detection of explosives by recyclable AIE luminogen-functionalized mesoporous materials
Dongdong Li, Jianzhao Liu, Ryan T. K. Kwok, Zhiqiang Liang, Ben Zhong Tang and Jihong Yu
Chem. Commun., 2012, Advance Article, DOI: 10.1039/C2CC31890C, Communication












 A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but
A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but