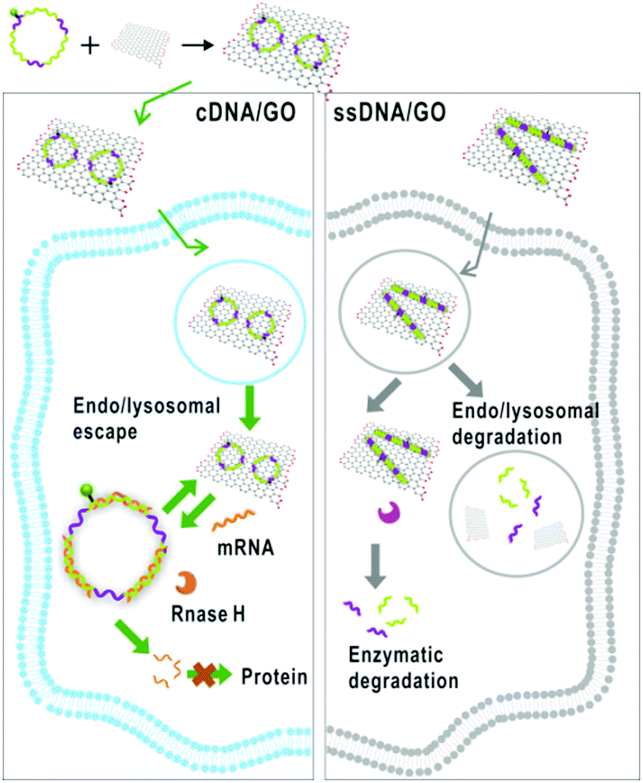
Scheme showing how cDNA/GO enters the cell and interacts with mRNA
Did you know that the combined length of DNA in your body’s cells is a number so large that the only references I could find use cosmic distances as a reference? Try twice the diameter of the solar system, or the distance to the moon and back 1500 times. Despite the complexity and infinite detail encountered when studying science, it is often something so simple as size that gives us pause. How can DNA be both uncomprehendingly huge and tiny at the same time?
The major function of DNA is to encode proteins, a process which begins with the transcription of genes into single-stranded messenger RNA (mRNA) molecules. It is mRNA that is directly translated into the strands of amino acids which fold to form proteins.
A team of researchers at Fuzhou University in China have developed a graphene oxide and circularised single-stranded DNA (cDNA/GO) hybrid material capable of penetrating living cells and binding mRNA. The material’s utility is shown in two practical applications: mRNA imaging and nucleotide therapeutics. The authors chose the mRNA of survivin and c-raf kinase as targets, because the enzymes are involved in carcinogenesis, and the mRNA are overexpressed in cancer cells and can be used as biomarkers.
cDNA was chosen for its increased stability over linear single-stranded DNA, which is rapidly degraded in vivo by exonucleases. For mRNA imaging the material is designed with a fluorescent dye coupled to the cDNA. GO was chosen as a hydrophilic delivery scaffold capable of adsorbing cDNA and quenching the dye. When cDNA/GO was incubated with HeLa cells (a cancer cell strain) a time-dependent increase in fluorescence was observed in the cytoplasm. Fluorescence is restored when cDNA encounters the target and desorbs from the GO to form a duplex with the mRNA.
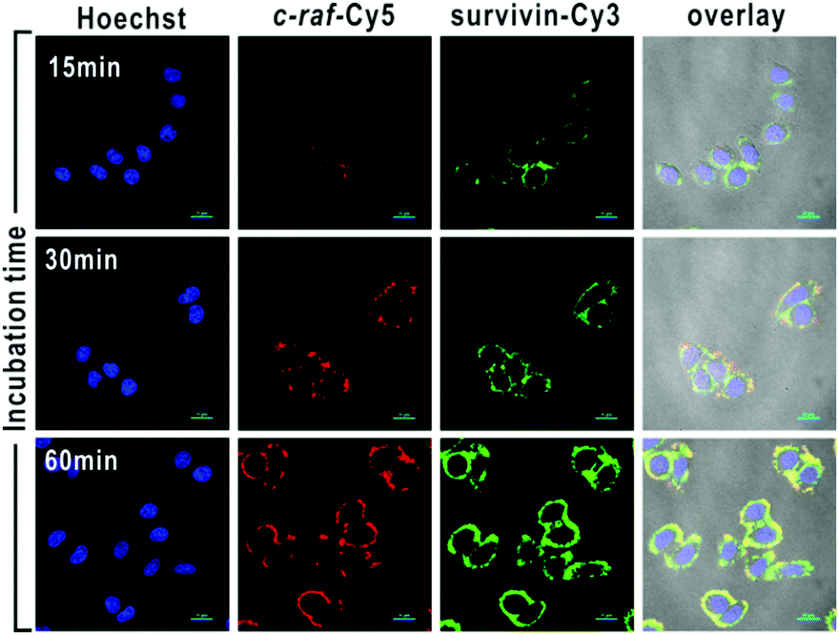
The mRNA of both survivin and c-raf kinase can be imaged in living cells with cDNA/GO.
The researchers also probed whether the material might serve as a therapeutic agent: if formation of the cDNA-mRNA duplex blocks translation it may reduce the load of c-raf kinase and survivin in the cell and influence cancer cell growth. Accordingly, the researchers found that when the HeLa cells were incubated with cDNA/GO, cell proliferation was inhibited in a dose-dependent manner.
This research contributes a robust design which can be applied to diverse mRNA targets because optimisable properties such as stability, bioavailability and selectivity are largely independent of the sequence of nucleotides.
To find out more please read:
Circular DNA: a stable probe for highly efficient mRNA imaging and gene therapy in living cells
Jingying Li, Jie Zhou, Tong Liu, Shan Chen, Juan Li and Huanghao Yang
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C7CC08906F
About the author
Zoë Hearne is a PhD candidate in chemistry at McGill University in Montréal, Canada, under the supervision of Professor Chao-Jun Li. She hails from Canberra, Australia, where she completed her undergraduate degree. Her current research focuses on transition metal catalysis to effect novel transformations, and out of the lab she is an enthusiastic chemistry tutor and science communicator.












 Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at
Tianyu Liu obtained his Ph.D. (2017) in Physical Chemistry from University of California, Santa Cruz in United States. He is passionate about scientific communication to introduce cutting-edge research to both the general public and scientists with diverse research expertise. He is an online blog writer for Chem. Commun. and Chem. Sci. More information about him can be found at