Chemists working at the University of Cambridge in the UK have discovered a new dicopper complex capable of forming diverse supramolecular structures.
The creation of supramolecular structures for a wide variety of uses has been something that chemists have pursued for years and, as such, new methods to access these structures are constantly sought. Jonathan Nitschke and colleagues are prominent figures in this field and have just reported a new copper complex that self assembles into larger architectures.
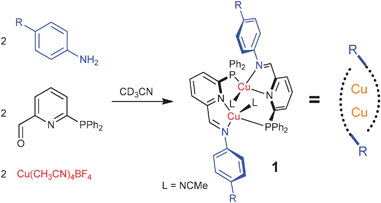
The team first assembled the copper complex and then showed they could modified it at two points. This is done either by substitution of solvent molecules with ligands capable of linking multiple complexes or by modifying the imine bonds. Using both these approaches and varying the ligands, it was possible to achieve the templated synthesis of 26- and 52-membered macrocycles. More complex assemblies that utilise this approach are currently being investigated.
Interested in finding out more? Then download Nitschke’s ChemComm article for free today. Also why not check out Dr Nitschke’s mini review and edge article form our sister journal Chemical Science?
Reactivity modulation in container molecules
Boris Breiner, Jack K. Clegg and Jonathan R. Nitschke
Chem. Sci., 2011, 2, 51-56
DOI: 10.1039/C0SC00329H, Minireview
Selective anion binding by a “Chameleon” capsule with a dynamically reconfigurable exterior
Yana R. Hristova, Maarten M. J. Smulders, Jack K. Clegg, Boris Breiner and Jonathan R. Nitschke
Chem. Sci., 2011, 2, 638-641
DOI: 10.1039/C0SC00495B, Edge Article












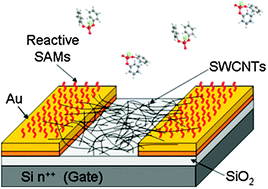 A new sensor for detecting nerve agents has been developed by scientists in France.
A new sensor for detecting nerve agents has been developed by scientists in France. Chinese researchers have shown for the first time that nanomaterials made from titanium dioxide (TiO2) can be used in cigarette filters to significantly reduce the amount of harmful chemicals inhaled by smokers. They say it offers a cheaper and safer alternative than using carbon-based nanomaterials and show potential for use in other filtering devices including gas masks and air purification systems.
Chinese researchers have shown for the first time that nanomaterials made from titanium dioxide (TiO2) can be used in cigarette filters to significantly reduce the amount of harmful chemicals inhaled by smokers. They say it offers a cheaper and safer alternative than using carbon-based nanomaterials and show potential for use in other filtering devices including gas masks and air purification systems.