Polydiacetylene polymers (PDAs) are a popular research topic for polymer and materials chemists due to their interesting optical, spectral, electronic and structural properties. Jong-Man Kim and colleagues’ recent Feature Article gives a detailed overview of the diverse range of structural morphologies and the related functional properties featured by PDAs in recent years and highlight their importance in sensor and display technologies.
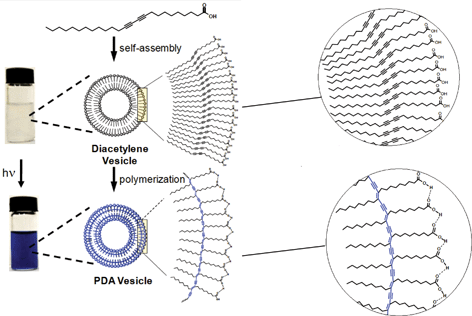
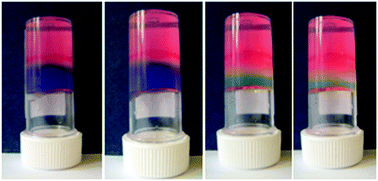
Interactions between the diacetylene (DA) monomers before polymerisation can directly influence the final polymerised structure. The monomers can be functionalised to contain motifs that encourage non-covalent interactions such as hydrogen bonding, π-stacking, electrostatics and hydrophobic interactions, allowing the DAs to self-assemble into nanostructures. Subsequent polymerisation of the acetylene groups results in cross-linking within the nanostructure, forming new materials with striking properties. One particularly interesting example of this is shown below – the DA monomers are substituted with long hydrophobic chains and polar head groups which assemble in water to form vesicles. Shining UV light on the vesicles causes the diacetylene groups to polymerise, generating PDA vesicles which are blue in colour.
For an in-depth and fascinating overview of the recent conceptual and technological advances in the chemistry of PDAs, download the full Feature Article here.
Posted on behalf of Cally Haynes, web science writer for ChemComm.











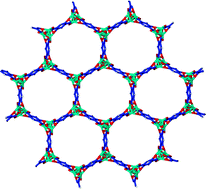 Xenon is naturally present in very small amounts in the atmosphere but radioactive forms are released following nuclear detonations, reprocessing and explosions, such as the recent catastrophe at Fukushima Daiichi Nuclear Power Plant in Japan. Xenon is also used in a variety of other applications, from lighting to medical imaging, so capturing and separating it (from its sister noble gas krypton) is important for both commercial uses and atmospheric monitoring.
Xenon is naturally present in very small amounts in the atmosphere but radioactive forms are released following nuclear detonations, reprocessing and explosions, such as the recent catastrophe at Fukushima Daiichi Nuclear Power Plant in Japan. Xenon is also used in a variety of other applications, from lighting to medical imaging, so capturing and separating it (from its sister noble gas krypton) is important for both commercial uses and atmospheric monitoring.