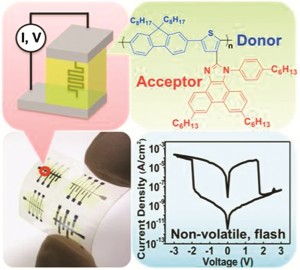
Scientists in Taiwan have made a flexible memory device, which they say could open up a new design approach for high performance flexible non-volatile resistive memory devices. Non-volatile devices are computer memory devices that can retain stored information even when not powered, for example read-only memory, flash memory, hard drives and floppy disks.
The team’s device consists of a single-layer donor-acceptor conjugated polymer fabricated on plastic polyethylene naphthalene. It displayed a low threshold voltage (±2V), low switching power (~100µW cm-2), large on/off memory window (104), good retention (>104s) and excellent endurance against electrical and mechanical stimuli, they say.
Link to journal article
Poly(fluorene-thiophene) Donor Tethered Phenanthro[9,10-d]imidazole Acceptor for Flexible Nonvolatile Flash Resistive Memory Devices
H-C Wu et al
Chem. Commun., 2012, DOI: 10.1039/c2cc34257j











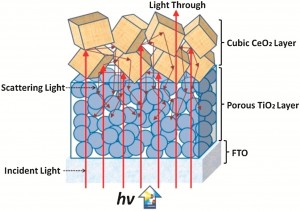
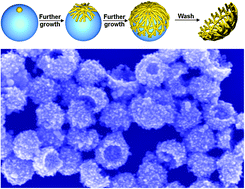 A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but
A gold nanocup – it sounds like something a posh fairy might drink out of. But actually, metal nanocups are promising particles for sensing and nanoelectronics thanks to their plasmon coupling and light scattering properties. Until now, they have been difficult to make but  3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?
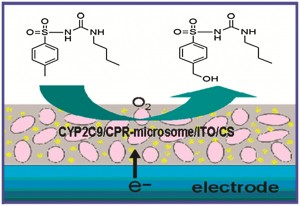
3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?